Bioxytran Reports Positive Study Results for a Broad-Range Antiviral Drug in Mild-to-Moderate COVID-19
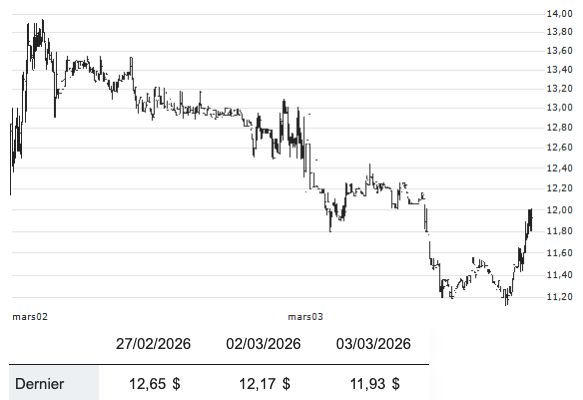
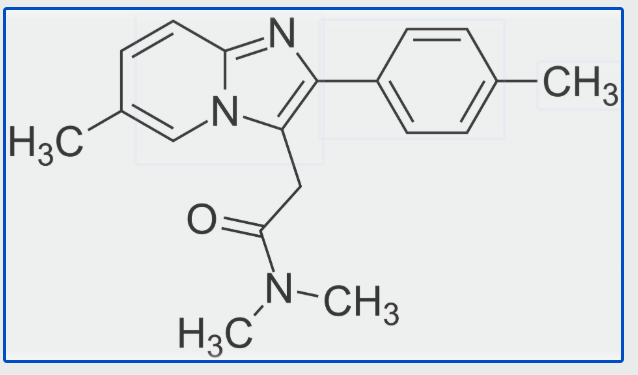
Bioxytran, Inc. recently announced promising results from a randomized, double-blind, placebo-controlled Phase 1b/2a clinical study of oral ProLectin-M in hospitalized patients with mild to moderate COVID-19. The study demonstrated that the highest evaluated dose of ProLectin-M (16,800 mg/day) led to statistically significant earlier viral clearance and faster clinical improvement by Day 5 compared to placebo,…