CRL Regulatory Shock Trades: How FDA Letters Move Billions Before Lunch
No fireworks. A 7:12 a.m. line to the exchange: “received an FDA complete response letter.” No press conference. No courtroom opera. Ten minutes later, the tape is bleeding. By lunch, whole business models have been repriced.
CRL means one thing, plainly: not approved as filed. But the reasons split. Two kinds of trouble surface in the paperwork. The story, and the plumbing. Evidence versus process. Either the trial doesn’t convince—not adequate and well-controlled, no substantial evidence. Or the machinery stumbles—manufacturing, controls, documentation, a vendor’s line going sideways.
Markets read that split fast. Evidence risk questions the molecule. Process risk taxes the calendar and the budget. One kills multiples. The other rents them for a while.
Watch the shape of the day. The gap at the open. The argument by the close. The verdict five days out. It repeats. Different tickers, same choreography.
Tell-tales hide in the middle of a paragraph: new adequate study required versus additional CMC information, no new trials requested. The first sounds like years. The second, like months. That difference is the price tag—sometimes a cliff, sometimes a detour.
Two kinds of “no” — two different price paths
When the message is fundamentally clinical — “not adequate and well-controlled,” “lack of substantial evidence” — the market reprices the net present value of the asset. The selloff is usually blunt and it sticks.
July 22, 2025 — Replimune. Disclosed a CRL for oncolytic virus RP1 in melanoma; the pivotal was described as “inadequate and not well-controlled,” not providing substantial evidence of effectiveness. Shares fell ~75% day-0. Not beta — a valuation reset of the program’s odds.
July 11, 2025 — Capricor. FDA declined deramiocel in DMD cardiomyopathy; premarket ~−50%, first full session ~−33% as language pointed to insufficient efficacy and an unreadable CMC section. The tape heard “new evidence required,” not “minor edits.”
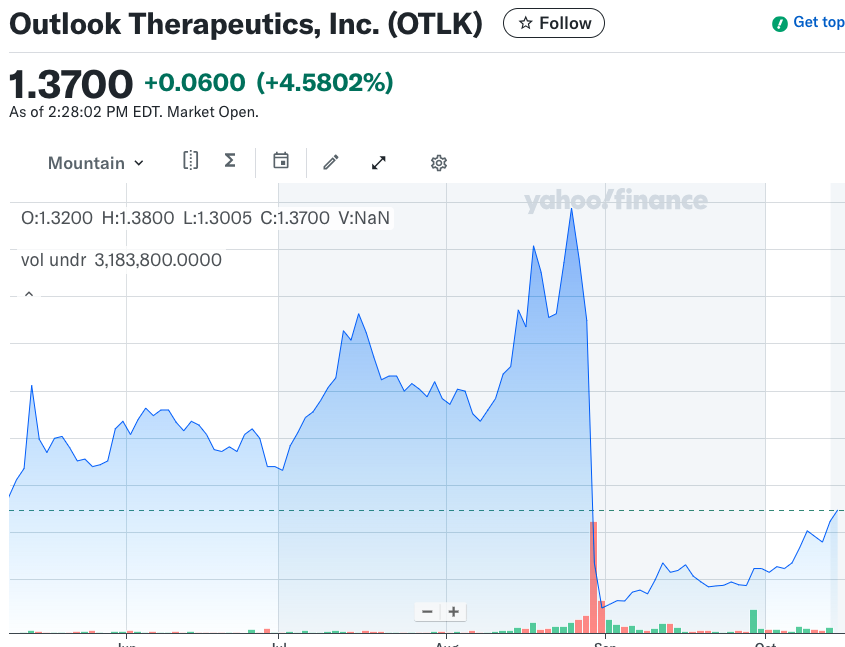
Aug 28, 2025 — Outlook Therapeutics. Second CRL for ONS-5010 (wet AMD), explicitly citing lack of substantial evidence. Shares slumped nearly 70% pre-market — classic clinical CRL tape.
Aug 19, 2025 — PTC Therapeutics. CRL for vatiquinone (FA) asking for another adequate, well-controlled study. Day-0 reaction muted as expectations were low and focus shifted to a recent approval elsewhere — portfolio context dulled a clinical blow.
When the problem is CMC/process and no new trials are requested, day-zero damage is often smaller and more reversible, because the market discounts time and cost, not probability of success.
Jun 28, 2024 — Rocket Pharma. BLA for Kresladi drew a CMC-focused CRL. Shares plunged ~14% intraday, then clawed back to ~flat/up by the close as investors reframed it as “addressable.” The dump-to-close rebound is a CMC tell.
Jul 11, 2025 — Ultragenyx. CMC CRL for UX111 (MPS IIIA). After-hours ~−5%. Messaging emphasized manufacturing/facility rather than efficacy. The tape priced a delay, not a thesis break.
When process morphs into evidence
Sometimes a “process” letter behaves like “clinical.” On Jan 16, 2025, Atara revealed a CRL driven by third-party manufacturing findings. The stock dropped >50% intraday and ~−40.5% by the close; within days came a program hold, turning a “fix the plant” story into pipeline risk. That is exactly when CMC ≈ clinical on price.
The severity ladder you can read in minutes
Labeling / technical clean-up. Small shock; quick resubmission.
CMC (sponsor-controlled). Moderate hit; partial reversal by the close; months to fix.
CMC (third-party / inspection heat). Can behave like clinical if OAI/WL/hold risk is in the air.
Clinical / design / inadequate evidence. Big, durable discount; often years to repair.
Notice how the vendor signal matters. On Sep 23, 2025, Scholar Rock flagged fill-finish issues at Catalent Indiana (now Novo Nordisk). Premarket slipped, but debate turned to remediation timelines and Type A scheduling — process risk, not a failed drug. A week later the same backdrop darkened as press noted an OAI classification at the site; the stock wobbled again. The difference between “addressable CMC” and “inspection escalation” can be a few words and a few percentage points.
On Oct 1, 2025, Fortress Biotech reported a CRL for CUTX-101 (Menkes) citing cGMP deficiencies at the manufacturing facility; shares fell ~30–36% that morning. The company stressed no efficacy/safety deficiencies — investors marked delay and CAPA cost, not asset failure.
How the shock actually unfolds on Day-0
It starts in the dark. Premarket is thin; one line in an 8-K or press note hits the wire and there isn’t much resting liquidity to absorb it. Bids vanish. A few headline-reading algos fire first — they’re fast, not wise — and the book gaps lower. That’s the mechanical part.
Then the microstructure takes over. The opening auction prints and sets an anchor everyone will argue with for the next six hours. Market makers widen. Options desks yank implied vol higher and skew goes crooked; delta-hedging adds forced sells into a falling tape. Risk models at funds flip from “event pending” to “ex-ante miss” and start cutting without reading a single paragraph. If the move is violent you may even get a LULD pause; that just reloads the spring.
Now the fundamental money shows up. Humans with PDFs and context. They hunt for three phrases and a timeline. If they see “not adequate and well-controlled,” “lack of substantial evidence,” “new study required,” that’s NPV risk — the molecule itself is in doubt. These days gap and keep bleeding; lower lows into the close as each incremental read points to years, not months.
If they see “additional CMC information,” “manufacturing deficiencies,” “no new trials requested,” “addressable,” and a hint of a Type A meeting or resubmission window, that’s timing risk — a DCF tax on the calendar. These days often dump and reverse. The open is panic; the close is an argument. Add control over the site and a decent runway, and the V-shape becomes the base case.
And if it’s CMC but the fix sits at a third-party with a hot inspection history or the letter smells like it could bleed into a hold, it trades like clinical. The first bounce dies. The pattern turns from V into gap-and-bleed because nobody can time a vendor’s remediation or the agency’s re-inspection cycle.
You can map Day-0 almost by the clock. Pre-open or after-hours headlines set direction, not magnitude. Open to first hour is price discovery. Late morning is when shapes diverge as the first coherent notes land. Midday drift tests the anchor: add detail and process risk compresses; hide behind boilerplate and it expands. Final hour is re-hedging and T+1 positioning. True clinical “no” usually closes on the lows; “plain CMC” that’s fixable often closes well off the lows. Third-party CMC with inspection heat tends to slide lower left to lower right.
V-shape. Clean CMC with control and a timeline. Panic exhausts, adults buy, the close recovers.
Flat-down. Light CMC/technical. The market taxes the calendar, not the molecule. Contained damage.
Gap-and-bleed. Clinical/design, or CMC that behaves like clinical (third-party + escalation risk). Every new paragraph confirms a long road; longs de-risk into the close.
This isn’t about algos versus people. It’s about sequencing. Machines move the price; language decides where it settles. Change three words — “no new trials requested” — and you change the day’s geometry. Add one clause — “third-party site under official action” — and you erase the bounce. Day-0 isn’t random; it’s a grammar test the market grades in real time.
A dozen live cases, one pattern
We intentionally mixed causes, sponsors, and sizes. The results rhyme.
Replimune (REPL). Clinical/design → −~75% day-0. “Inadequate and not well-controlled.” ir.replimune.com • Barron’s
Capricor (CAPR). Clinical/efficacy → premarket −50%, ~−33% by close. Reuters
Outlook Therapeutics (OTLK). Clinical/efficacy → ~−70% premarket. Second CRL. Reuters
PTC Therapeutics (PTCT). Clinical/“new adequate study” → muted day-0 (portfolio cushion). Reuters • BioWorld
Atara (ATRA). CMC/third-party, followed by program hold → >−50% intraday, ~−40% close. Reuters
Scholar Rock (SRRK). CMC/vendor (Catalent) → low-teens premarket drop; debate centers on remediation timeline. Reuters
Rocket (RCKT). CMC/info request → ~−14% intraday, rebound by close. ir.rocketpharma.com
Ultragenyx (RARE). CMC/facilities → ~−5% after-hours; framed as addressable. ir.ultragenyx.com
Fortress Biotech (FBIO). CMC/plant deficiencies → ~−30–36% morning; “no efficacy issues” flagged. Reuters • Morningstar
Biogen (BIIB), high-dose nusinersen sNDA. CMC module update CRL → technical path to refile; day-0 reaction modest. TradingView
Ten cases are enough to see the contour: clinical letters behave like permanent NPV edits; plain-vanilla CMC looks like delay-discounts with a decent chance of t+5 stabilization if the sponsor controls the fix and cash runway isn’t tight.
Read the letter, hear what the market hears
Clinical language. “Not adequate and well-controlled,” “lack of substantial evidence,” “new study required” → expect gap-and-stick and a bearish t+5 absent a novel data plan. (Replimune, Outlook, PTC.)
Process language. “Additional CMC information,” “manufacturing deficiencies,” “addressable,” “no new trials requested” → expect intraday volatility and partial close recovery, with the story shifting to Type A timing and CAPA. (Rocket, Ultragenyx, Scholar Rock, Fortress.)
Third-party heat. Named vendors, recent OAI/WL/483 → upgrade the risk; CMC can trade like clinical if inspections escalate or control is weak. (Atara; watch Catalent Indiana for SRRK.)
What actually gets priced
Probability of success (clinical/design). When evidence is questioned, success odds fall hard. The asset’s expected value shrinks; multiples compress.
Time to cash flow (CMC delays, resubmission cadence). Process gaps push launches right. DCF punishes waiting; a clear Type A → resubmission path limits the discount, vagueness enlarges it.
Cost of the fix and financing risk (runway, dilution, partner support). Remediation is CAPA, validation, stability, re-inspection. With runway it reads as opex; with thin cash it reads as dilution. Control over the site is the hinge.
That’s why clinical CRLs dump the multiple and CMC letters mostly move timing and opex. It’s also why a CMC-only letter can still crater a stock when third-party control is thin or hold risk looms.
Playbooks — What to Do When a CRL Hits
Investor view. When the news says the FDA’s letter is “addressable,” that usually means it’s a technical or manufacturing issue, not a rejection of the drug itself. If the company also says “no new trials required,” that’s a clue the science is still valid. Add in signs that the company has at least a year of cash and a planned meeting with FDA (a “Type A” meeting), and the situation often looks like a delay, not a disaster. Stocks that fall sharply on such letters sometimes recover once investors realize it’s a matter of fixing paperwork or production, not rerunning years of studies.
But if there’s mention of deeper regulatory trouble — like a warning to a contract manufacturer, a product hold, or an FDA classification such as “Official Action Indicated (OAI)” — that’s different. Those cases can take much longer to clean up, and markets treat them almost like failed clinical results. The rule of thumb: the more control the company has over the fix, the faster the recovery; the less control, the longer the pain.
Company view (IR / QA). How a firm explains a CRL can decide how severe the market reaction becomes. The message should say clearly what the FDA did *not* find: for example, “no safety or efficacy concerns,” or “no new trials requested.” Then, specify what happens next — what corrective actions (CAPA) are planned, and roughly when the next meeting or resubmission will take place. When companies stay vague, investors assume the worst. Clarity about timing, cost, and scope can limit the selloff.
How We Looked at the Data
For each company, the “event day” starts when the CRL announcement first appears — whether it’s a pre-market press release or an after-hours SEC 8-K. We tracked the stock from that moment through the market close and then for the next five trading days. The analysis compares those moves to a benchmark like the S&P 500 or Nasdaq to find the *abnormal return* — the move that can’t be explained by the general market trend. Each case is labeled by type: Clinical/Design (evidence not convincing), CMC/Vendor (manufacturing or process issues), or Labeling/Other. If a third-party site or inspection problem appears, we flag it, because those often turn a short-term technical delay into a longer, costlier setback.
The Takeaway
The market doesn’t panic because a company hears “no” from the FDA — it panics because it can’t tell *which kind* of “no” it is. A letter that questions the evidence behind a drug can erase years of projected revenue; one that asks for better documentation can mean only a few extra months and extra spending. The difference shows up in how the stock moves that day. In 2024–2025, that single distinction — science versus process — explained why some charts plunged and stayed down, while others dropped fast, stabilized, and found buyers again by the close.
Use the database as your supply chain compass →