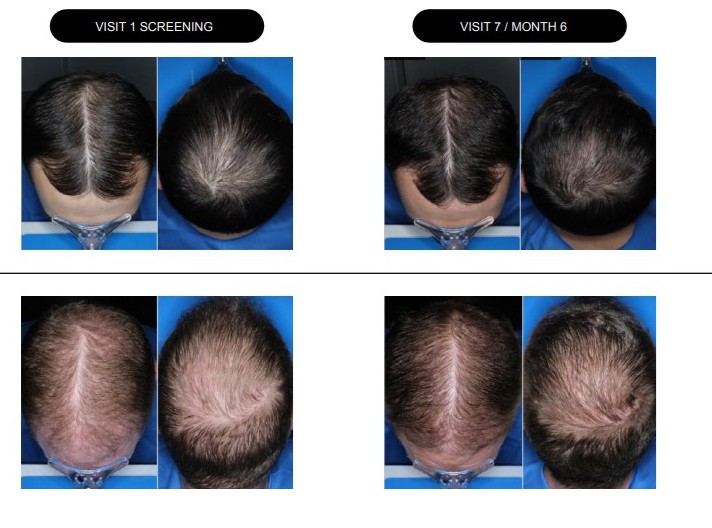
Cosmo Pharmaceuticals (SIX Swiss: COSM) has experienced a remarkable surge in its stock price, climbing nearly 40% after releasing topline Phase III data for its clascoterone candidate, aimed at treating androgenetic alopecia (AGA). This data, which Cosmo describes as compelling, comes from two identically designed trials that enrolled a total of 1,465 patients, marking it as the largest Phase III program for any topical AGA candidate to date.
The results showed a combined 252% relative improvement in Target-Area Hair Count (TAHC) for clascoterone compared to placebo, with the SCALP 2 trial reporting an impressive 539% improvement. This strong performance has not only garnered investor interest but also positions Cosmo strategically within the lucrative AGA market, which the company estimates could exceed $20 billion in the U.S. alone.
As Cosmo prepares to present detailed findings at a medical conference and in peer-reviewed journals, the company is also working on completing 12-month safety data, expected in spring 2026. Should these results remain positive, Cosmo plans to file for regulatory approvals with the FDA and EMA, potentially reshaping the landscape for AGA treatments.
Start your 7-day trial and see what the database can do →