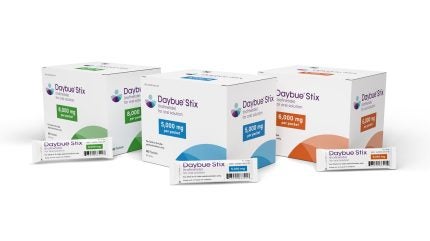
The US Food and Drug Administration (FDA) has granted approval for Acadia Pharmaceuticals’ Daybue Stix (trofinetide), marking a significant milestone in the treatment of Rett syndrome. This condition, which primarily affects females and leads to severe cognitive and physical impairments, has long lacked effective therapeutic options. The approval of Daybue Stix comes after a rigorous review process, highlighting the drug’s potential to address unmet medical needs in this patient population.
This development not only underscores the FDA’s commitment to advancing treatments for rare diseases but also reflects the growing interest in neurological disorders within the pharmaceutical industry. As more companies pivot towards developing specialized therapies, the approval of Daybue Stix may encourage further investment and innovation in the field. Stakeholders in regulatory, quality assurance, and supply chain sectors will need to adapt to the evolving landscape as new therapies emerge, ensuring compliance and quality in the delivery of these critical treatments.
Use the database as your supply chain compass →