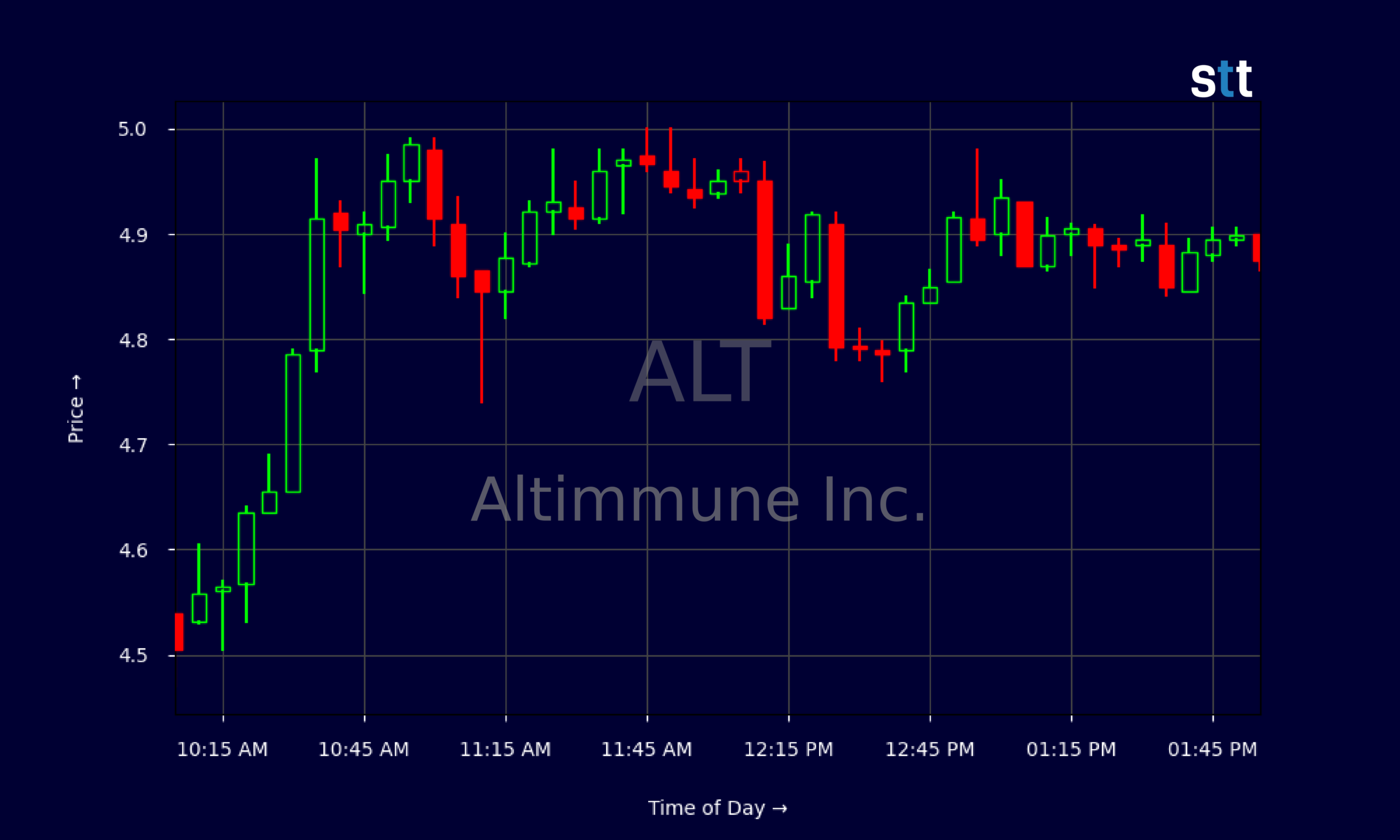
Altimmune has announced its advancement to Phase 3 trials for pemvidutide, a GLP-1/glucagon agonist aimed at treating metabolic dysfunction associated with steatohepatitis (MASH). This decision comes on the heels of a disappointing Phase 2 trial result earlier this year, which raised concerns about the drug’s efficacy. However, the company has identified a longer treatment duration that may enhance the drug’s potential effectiveness, prompting this new phase of clinical evaluation.
This strategic pivot highlights the ongoing challenges in the obesity and metabolic disorder markets, particularly as regulatory scrutiny intensifies. The implications for Altimmune are significant; a successful Phase 3 trial could position pemvidutide as a leading therapeutic option in a competitive landscape. As the company seeks to regain investor confidence, the outcome of this trial will be closely monitored by industry professionals and stakeholders alike.
Get started today with Solo access →