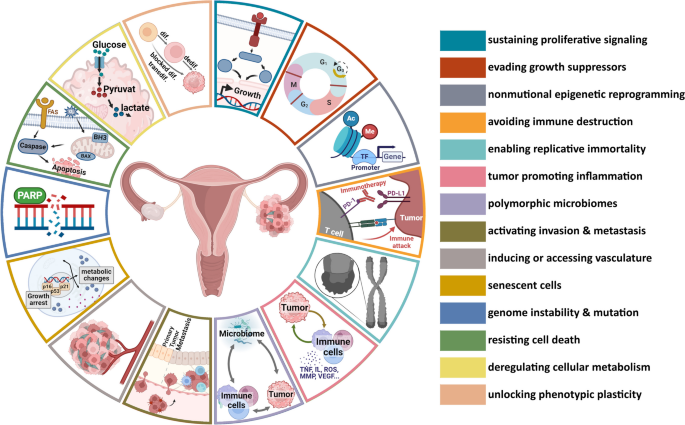
BioNTech has announced that its leading antibody-drug conjugate (ADC) has successfully completed a Phase 3 trial in China, targeting patients with hard-to-treat breast cancer. This significant achievement not only underscores the potential of the ADC in addressing challenging oncological cases but also heightens expectations for the ongoing global late-phase trial of the same drug.
The successful trial results in China are pivotal, as they may influence regulatory pathways and market access strategies for the ADC in other regions. The growing emphasis on personalized medicine and targeted therapies in oncology makes this development particularly relevant for stakeholders across the pharmaceutical landscape, from regulatory bodies to quality assurance teams.
As BioNTech prepares to leverage this momentum, the implications for sourcing, manufacturing, and portfolio management become increasingly critical. The ADC’s performance could set a precedent for future collaborations and innovations in the field, positioning BioNTech and DualityBio as key players in the competitive landscape of cancer therapeutics.
Open the full market picture for your next decision →