Lipid nanoparticles (LNPs) have emerged as a pivotal advancement in drug delivery, particularly highlighted during the COVID-19 pandemic as vehicles for mRNA vaccines. Their ability to deliver nucleic acid cargo safely and effectively has garnered significant attention and investment, underscoring their potential in modern medicine.
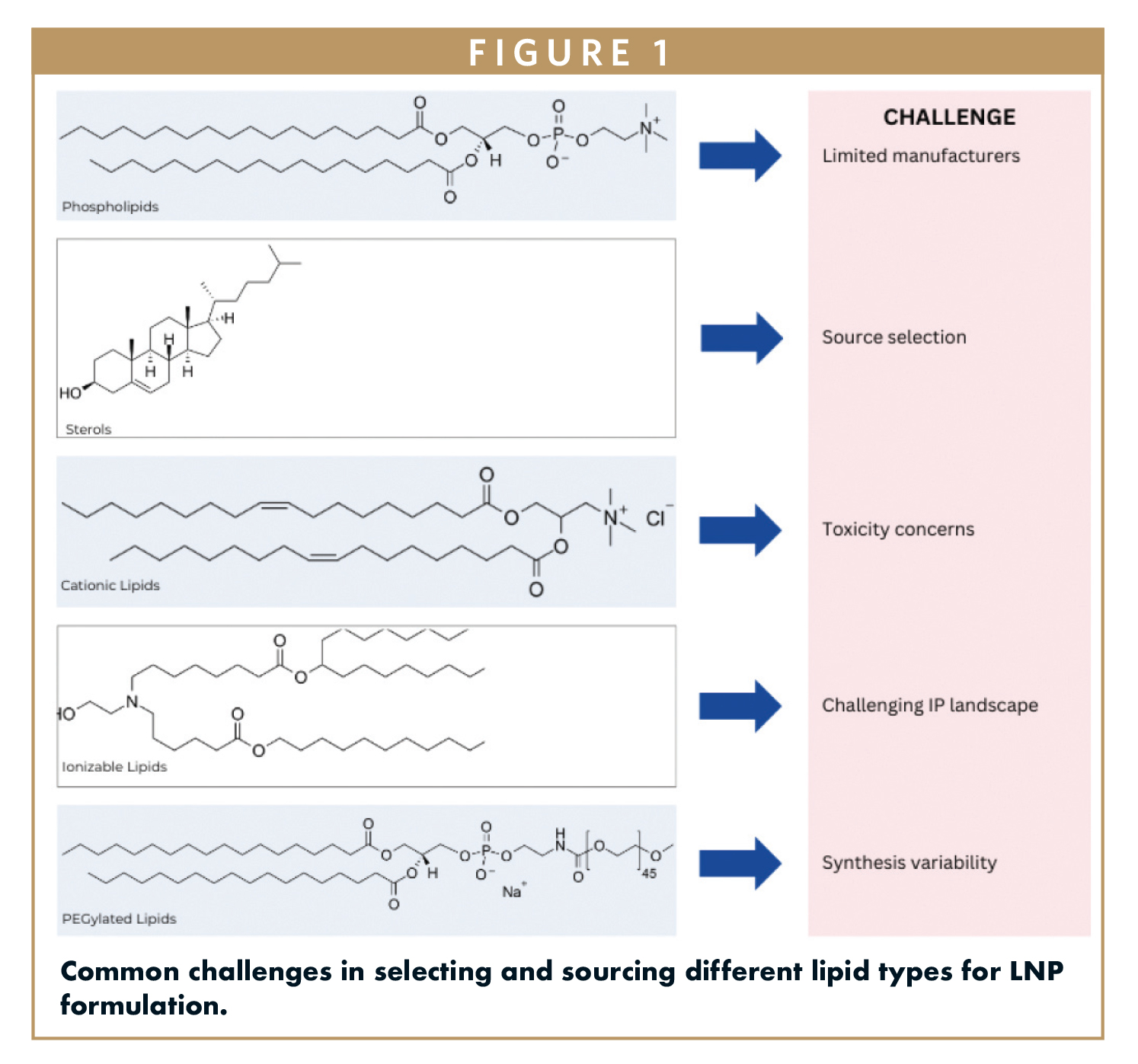
However, the development of successful LNP-based therapies is fraught with challenges, particularly in lipid selection and sourcing. The choice of lipids directly influences critical factors such as particle size, encapsulation efficiency, and cellular uptake. Selecting the right lipids is essential for optimizing stability and efficacy, yet the complexity of the lipid landscape can introduce significant hurdles. Variations in lipid composition can lead to drastic functional changes, necessitating a careful and informed approach to lipid sourcing.
Moreover, the therapeutic success of LNP formulations hinges on the ability to produce lipids consistently at high purity levels. Even minor impurities can compromise safety and efficacy, making rigorous quality assurance paramount. As companies transition from research to clinical production, strategic planning in lipid sourcing becomes crucial to avoid delays and ensure compliance with regulatory standards. Engaging suppliers with expertise across all stages of lipid development can mitigate risks and streamline the path to successful LNP therapies.
Use the database as your supply chain compass →