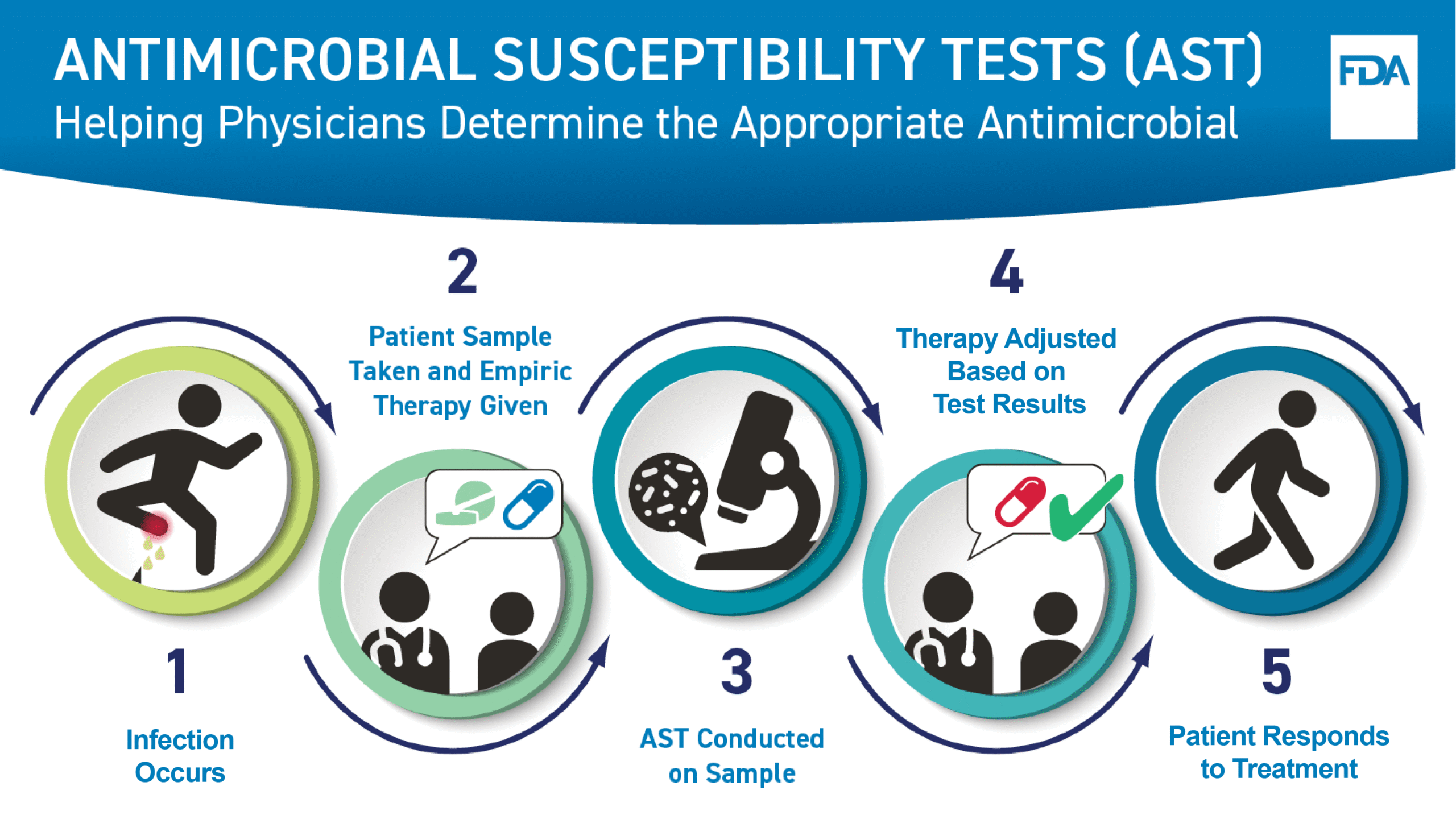
The FDA has announced updates to its STIC (Scientific and Technical Information Center) webpages, specifically focusing on antibacterial and antifungal drugs. This development reflects the agency’s ongoing commitment to enhance transparency and accessibility of critical information regarding drug development and regulatory processes.
These updates are significant as they provide stakeholders in the pharmaceutical industry, including regulatory affairs, quality assurance, and sourcing professionals, with the latest guidance and data essential for compliance and innovation. The revisions aim to streamline access to vital resources that can influence decision-making in drug development and approval pathways.
As the landscape of antimicrobial resistance continues to evolve, these updates serve as a timely reminder of the FDA’s role in facilitating the development of effective therapeutic options. Industry professionals must stay informed of these changes to ensure alignment with regulatory expectations and to leverage new insights for strategic planning.
Use the database as your supply chain compass →