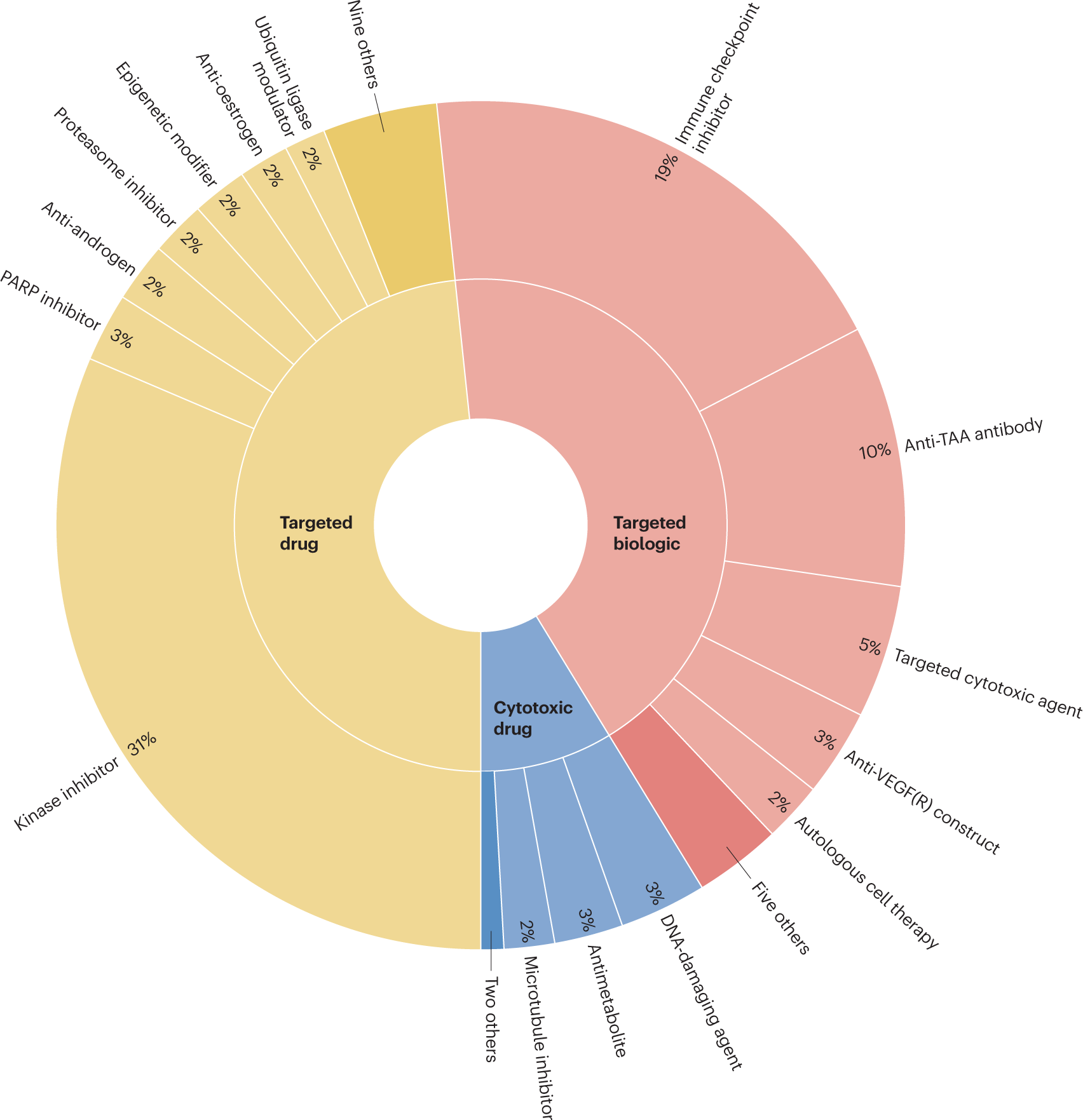
The FDA has announced that it will not issue approval announcements for every drug approval or label update within the oncology and hematology sectors. This selective notification process underscores the agency’s focus on significant approvals that may impact treatment protocols and patient outcomes.
This approach raises questions about transparency and accessibility of information for healthcare professionals involved in regulatory affairs, quality assurance, and clinical management. As the oncology and hematology fields continue to evolve with rapid advancements in therapies, the lack of comprehensive announcements could hinder timely awareness of new treatment options and updates.
Industry professionals must rely on resources like Drugs@FDA for the latest approvals and prescribing information. This shift emphasizes the need for stakeholders to actively monitor regulatory updates to remain informed and competitive in a fast-paced market.
Use the database as your supply chain compass →