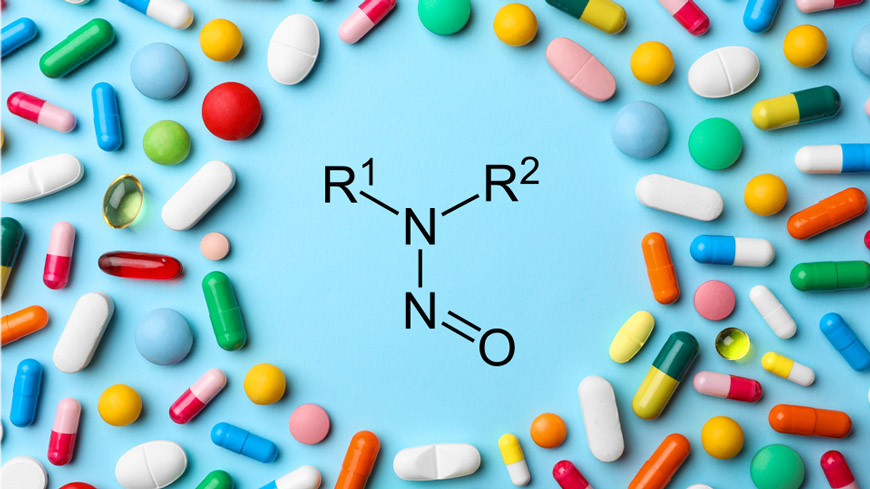
The story really does begin in a hot stretch of 2025, when three regulators – EMA (European Medicines Agency), TGA (Therapeutic Goods Administration, Australia), and Health Canada – moved almost in concert. In early July, EMA published a dry, businesslike post-mortem on the first five years of the “nitrosamine crisis” and, just as importantly, drew a route for the next five: CPCA (Carcinogenic Potency Categorisation Approach) as the standard engine, EAT (Enhanced Ames Test) as the “pressure-relief valve,” and a living Appendix-1—a list of AI (Acceptable Intake) values that is refreshed whenever science turns the page. By the end of the month, Australia’s TGA essentially said “we’re with them,” directing sponsors to AI tables aligned with the latest EMA updates. And on August 1, Canada replaced ambiguity with concreteness—clocks and spreadsheets: it added 17 nitrosamines with AIs, revised at least one well-known case, and wrote down exactly how much time you have to fix things once a new AI appears. This was the moment when “firefighting mode” turned into rules of the road.
Now add a fourth player – FDA (Food and Drug Administration, U.S.). Two years earlier, FDA had released a separate final guidance on NDSRIs (Nitrosamine Drug Substance-Related Impurities)—RAIL—and in September 2024 updated the big base nitrosamine control guidance (Rev.2). And in 2024–2025, the agency nailed a living CDER web page to this pair, where it promptly posts: (1) recommended AIs by CPCA, (2) AIs based on compound-specific or read-across assessment, (3) interim AIs under supply-shortage risk, (4) implementation timelines, (5) emerging issues, (6) recommended analytical methods, and (7) safety tests. It’s not law, but it’s FDA’s “current thinking,” and for the U.S. that’s a set of road signs.
The mechanics without acronyms: what to explain to a CFO in 5 minutes
EMA. CPCA sorts nitrosamines into five “baskets” by carcinogenic potency with predefined AIs: 18, 100, 400, 1500, and 1500 ng/day for Categories 1–5 respectively. You start from these numbers if you don’t have stronger compound-specific data. Then comes the “valve”: a negative (GLP-compliant) Enhanced Ames allows control at 1.5 µg/day. This CPCA + EAT combination is woven into the European “map” and mirrored by neighbors, which means global portfolios should be designed for it, not argued with it.
FDA. The formal approach is the same – CPCA as default—but Category-1 numbers differ: FDA has 26.5 ng/day versus Europe’s 18 ng/day; for Categories 2–5 it’s 100, 400, and 1500 (for 4 and 5) ng/day. Pushing the limit up to 1500 ng/day, FDA proposes not only via EAT-negative, but also by adding a second in-vitro mammalian cell mutagenicity test and metabolism data (human hepatocytes/microsomes); for AI above 1500, in vivo may be requested. In other words, the philosophy is common, but the evidence package is stricter.
Canada. As of August 1, 2025: if an AI was in Appendix-1 before 2025-08-01, your CAPA (Corrective and Preventive Action) window closes 2028-08-01; for AIs published after that date, you have three years from publication. And no more “LTL AIs” (less-than-lifetime): all AIs are lifetime, including during CAPA. In parallel, Canada gives BE expectations (bioequivalence) for reformulations using nitrosation suppressors (antioxidants, pH control). These are not polite marginal notes; they’re direct instructions for CMC plans.
What exactly the regulators put down (and where to read)
EMA (July 2025). The EMRN (European Medicines Regulatory Network) showed how to be fast without being sloppy: it confirmed the Article 5(3) framework, the role of NIOG (Nitrosamine Implementation Oversight Group) as the overseer of implementation, and a shift from “one-off events” (sartans, ranitidine, early NDSRI scares) to a durable operating mode: CPCA when data are thin, compound-specific when data exist, and a living Appendix-1 so sponsors don’t have to guess. In the same breath came what industry had quietly hoped for: more than 170 AI values are already published, and the approach has kept patients safe without blanket product removals. That sentence appears only when the reviewers “have receipts.”
TGA (31 July 2025). Formally it looks like “cosmetics,” in essence it’s removing an axis of uncertainty: TGA explicitly states it is aligned with recent EMA updates and points sponsors to the current AI lists. For global CMC teams this means: Berlin and Brisbane no longer diverge in tactics.
Health Canada (1 August 2025). The refresh added 17 new entries to Appendix-1 and, just as important, nailed time to the wall: three-year CAPA windows, LTL closed, and BE expectations for “suppressor” reformulations spelled out.
FDA (CDER “live” tables).
— Table 1 (CPCA): a huge roster of “hypothetical” NDSRIs with categories and AIs (e.g., N-nitroso-desmethyl-amitriptyline—Cat.1 26.5 ng/day).
— Table 2 (compound-specific/read-across): for example, N-nitroso-desmethyl-diltiazem—100 ng/day (read-across to NNK), which contrast sharply with Canada’s 18 ng/day for its NDSRI—an important inter-regulatory delta for strategy.
— Table 3 (interim AI): when standard limits would break supply, FDA posts temporary values (revised review dates—through 2026-08-01 for several cases: MNP/rifampin, nitroso-fluoxetine, nitroso-nortriptyline, etc.).
— Table 4 (timelines): on 2025-06-23, FDA acknowledged not everyone would make 2025-08-01, and requested a progress update by 2025-08-01 with specifics (targeted forced degradation, methods/validation, batches/dates, results, root cause, mitigation attempts, and an estimated completion timeframe). This is contact oversight, not amnesty.
And what about EAT and “1.5 µg/day”?
— In EMA/EU, a negative EAT (under valid enhanced conditions) permits control at 1.5 µg/day—explicit in the Q&A/Appendix-3.
— FDA uses EAT as part of the package for raising AI and adds a required second in-vitro mammalian mutagenicity test plus metabolism data; for AIs above 1500, in vivo may be requested. This matters for planning the evidentiary strategy to lift the limit.
Where it hurts today: examples from Canada’s table (2025-08-01)
Open Health Canada’s Appendix-1 from August 1 and you’ll see Category-1 pressure points at 18 ng/day for amiodarone (N-nitroso-N-desethyl-amiodarone), diltiazem (N-nitroso-N-desmethyl-diltiazem), ropinirole (N-nitroso-N-despropyl-ropinirole), and even an NNTA entry for cobicistat—this is a real design challenge or ruthless SKU management. Then: brinzolamide—100 ng/day (Cat.2), carvedilol—400 ng/day (Cat.3)—painful but usually manageable with chemistry-of-design and targeted analytics. And the “sigh” column: norfloxacin, vancomycin, dalbavancin, nadolol—1500 ng/day (Cat.4/5), where, with a solid risk file plus EAT-negative, routine control is realistic. A small but telling revision: N-nitroso-N-desmethyl-doxylamine is now 100 ng/day, not the earlier 18—softening that anyone who has lived in the “18 ng/day trench” will notice. This is a “living list” that actually lives.
Compact slice (Canada, 2025-08-01):
| Related API | N-nitrosamine | CPCA | AI (ng/day) | Date |
|---|---|---|---|---|
| Amiodarone | N-nitroso-N-desethyl-amiodarone | 1 | 18 | 2025-08-01 |
| Diltiazem | N-nitroso-N-desmethyl-diltiazem | 1 | 18 | 2025-08-01 |
| Ropinirole | N-nitroso-N-despropyl-ropinirole | 1 | 18 | 2025-08-01 |
| Brinzolamide | N-nitroso-brinzolamide | 2 | 100 | 2025-08-01 |
| Carvedilol | N-nitroso-carvedilol | 3 | 400 | 2025-08-01 |
| Norfloxacin | N-nitroso-norfloxacin | 4 | 1500 | 2025-08-01 |
| Vancomycin | N-nitroso-vancomycin | 4 | 1500 | 2025-08-01 |
| Nadolol | N-nitroso-nadolol | 5 | 1500 | 2025-08-01 |
| Doxylamine | N-nitroso-N-desmethyl-doxylamine | — | 100 | 2025-08-01 |
Source: Appendix-1, Health Canada.
The August rumor mill: why rumors matter for strategy
In early August, practitioners flagged that two Category-1 newcomers—N-nitroso-doravirine dimer and N-nitroso-nirogacestat – had reportedly come out EAT-negative and were being controlled at 1.5 µg/day in EMA’s internal update streams. Until the Excel sheet posts, this is a strong signal, not canon; if confirmed, it proves a point many only half-believed: even Category 1 can escape the 18 ng/day vise if the biology is convincingly negative. Design dossiers so you have that exit ramp. (This comes from an industry forum; treat it as indicative, not as an official source.)
Who really loses in the “2.0” regime?
Not a class of molecules, but poor portfolio discipline: too many strengths/sites, formulations that invite nitrosation, and no credible story for why they’re safe.
— Category 1 at 18 (EMA/HC) or 26.5 (FDA) ng/day punishes noisy processes and weak material control.
— Categories 2–3 survive if you can show purge/prevention/LOQ with sensitivity that makes sense against AI.
— The 1500 ng/day cohort “coasts” only if you secure EAT-negative (and in the U.S.—add the second in-vitro test/metabolism). Regulators align on CPCA math and the 1.5 µg/day rule (with different evidentiary wraps); from here it’s your choice: engineer your way out, or re-profile your map of SKUs and markets.
FDA: three important blocks you cannot ignore
- Interim AI under shortage threats. The table currently lists MNP/rifampin, nitroso-fluoxetine, nitroso-nortriptyline, and others with review dates through 2026-08-01. Use them, but don’t hide behind them: FDA says clearly they’re temporary and will be revisited.
- Implementation timelines. Formally FDA wanted NDSRI confirmatory testing finished and changes submitted by 2025-08-01, but on 2025-06-23 it acknowledged not all will make it—and asked for a progress update with a tight checklist (forced degradation, method/validation, batches/date, results, root cause, mitigation attempts, completion estimate) by 2025-08-01, with targeted deadlines to be tuned afterward. This is contact control, not a free pass.
- Emerging issues. The freshest is leachable NDBA and other small nitrosamines from infusion bags (2025-08-18): this is not tied to a specific API and breaks the old model “nitrites only from excipients/process.” Check packaging scenarios for parenterals and long infusions.
Methods: how to test and how to “lift” AI
Analytics. FDA publishes a set of ready-made methods (GC-MS/MS, LC-HRMS, LC-ESI-HRMS, RapidFire-MS/MS) for specific “API/nitrosamine” pairs, but validation is on you if the data go into a regulatory submission. If your LOQ doesn’t meet the AI, you’ll need science-based justification.
Safety (how to justify a higher limit).
— EMA/EU: EAT-negative is a route to 1.5 µg/day (with GLP and the enhanced conditions laid out in Appendix-3).
— FDA: recommends enhanced Ames plus a second in-vitro mammalian mutagenicity test and in-vitro metabolism (hepatocytes/microsomes) to support 1500 ng/day; for >1500 it may ask for in vivo.
What a Marketing Authorisation Holder (MAH) should do right now
- Re-score the portfolio: anchor your top-revenue products to CPCA categories and the latest Appendix-1 (EU/Canada) and Tables 1/2 (U.S.). Pinpoint where FDA gives read-across 100 ng/day while Canada/Europe keep 18 ng/day (the diltiazem example). That’s your map of conflicts in multi-country supply.
- Sync with Australia: assume TGA reads the same lists as EMA; don’t create divergence in controls.
- For Canada: hard-code in QMS the dates 2028-08-01 and “+3 years from publication” for new AIs; do not apply LTL-AI; keep a BE plan ready for suppressor reformulations.
- For the U.S.: if you didn’t make 2025-08-01, submit the progress update per FDA’s checklist and push the chosen strategy (specification/reformulation/packaging change) toward the targeted deadlines that FDA promised to announce after analyzing the updates. Watch interim AIs: they live through 2026-08-01, but may be reconsidered earlier if shortage risk eases.
- EAT plan: treat EAT-negative as a strategic “exit,” but factor in differences between EU and U.S. in the volume of data for 1.5 µg/day. Design dossiers to serve both logics at once.
- Packaging stress-test for infusion forms: check leachables (NDBA and others)—this is a new front.
Mini-slice of FDA (to spot “where it doesn’t match”)
| N-nitrosamine | Source (API) | Basis | Recommended AI |
|---|---|---|---|
| N-nitroso-desmethyl-diltiazem | Diltiazem | Read-across to NNK | 100 ng/day |
| N-nitroso-duloxetine | Duloxetine | Read-across to NNK | 100 ng/day |
| NDMA | Multiple | Compound-specific | 96 ng/day |
| NDEA | Multiple | Compound-specific | 26.5 ng/day |
| N-nitroso-methylphenidate | Methylphenidate | Read-across to NPIP | 1300 ng/day |
Source: FDA CDER “Acceptable Intake Limits,” Tables 1–2. Re-check the current rows at filing.
The bottom line isn’t that limits got stricter or looser. The bottom line is that the rules got predictable.
European CPCA+EAT, TGA alignment, and Canada’s CAPA clocks mean the era of “surprises” has been replaced by an era of designed-in control. Some products will exit. Some will reformulate. Sometimes you’ll be saved by an EAT-negative – like a plot twist. But the leitmotif of summer 2025 is unambiguous: nitrosamines are no longer a crisis; they’re a discipline. And disciplined portfolios win.
Sources and for re-verification
EMA EMRN report (July 8, 2025): TTC 18 ng/day default, CPCA/EAT institutionalization, flexibility language, NIOG & international collaboration. European Medicines Agency (EMA)
EMA Q&A (latest rev): Principle that EAT-negative → 1.5 µg/day control. European Medicines Agency (EMA)
EMA “What’s new” feed: Appendix-1 update signals (watch for Rev. 11 spreadsheet drop). European Medicines Agency (EMA)
TGA update (31 Jul 2025): alignment and links to AU AI lists. Therapeutic Goods Administration (TGA)
Health Canada overview + Appendix-1 table (1 Aug 2025): CAPA timelines, LTL policy, 17 new entries, doxylamine revision; live AI table with sort/filter. Canada.ca
Signal (community) on EMA’s Aug additions/EAT outcomes for Doravirine dimer & Nirogacestat: use as an early-warning datapoint until the official spreadsheet posts. Nitrosamines Exchange
Glossary of abbreviations
| Abbreviation | Expansion |
|---|---|
| AI | Acceptable Intake |
| CPCA | Carcinogenic Potency Categorisation Approach |
| EAT | Enhanced Ames Test |
| NDSRI | Nitrosamine Drug Substance-Related Impurity |
| FDA | U.S. Food and Drug Administration |
| CDER | Center for Drug Evaluation and Research (FDA) |
| EMA | European Medicines Agency |
| CHMP | Committee for Medicinal Products for Human Use (EMA) |
| NIOG | Nitrosamine Implementation Oversight Group (EMA/EMRN) |
| EMRN | European Medicines Regulatory Network |
| TGA | Therapeutic Goods Administration (Australia) |
| HC | Health Canada |
| MAH | Marketing Authorisation Holder |
| CAPA | Corrective and Preventive Action |
| QMS | Quality Management System |
| LOQ | Limit of Quantitation |
| BE | Bioequivalence |
| OECD TG 471 | OECD Test Guideline No. 471 — Bacterial Reverse Mutation Test (Ames) |
| GLP | Good Laboratory Practice |
| TTC | Threshold of Toxicological Concern |
| GC-MS/MS | Gas Chromatography–Tandem Mass Spectrometry |
| LC-HRMS / LC-ESI-HRMS | Liquid Chromatography–High-Resolution Mass Spectrometry (incl. ESI) |
| NDMA / NDEA / NPIP / NPZ / NNK | Reference nitrosamines/surrogates in FDA assessments |
| RAIL | Recommended Acceptable Intake Limits for NDSRIs (FDA guidance) |
| LTL | Less-than-lifetime (in Canada not applied) |
Start your 7-day trial and see what the database can do →