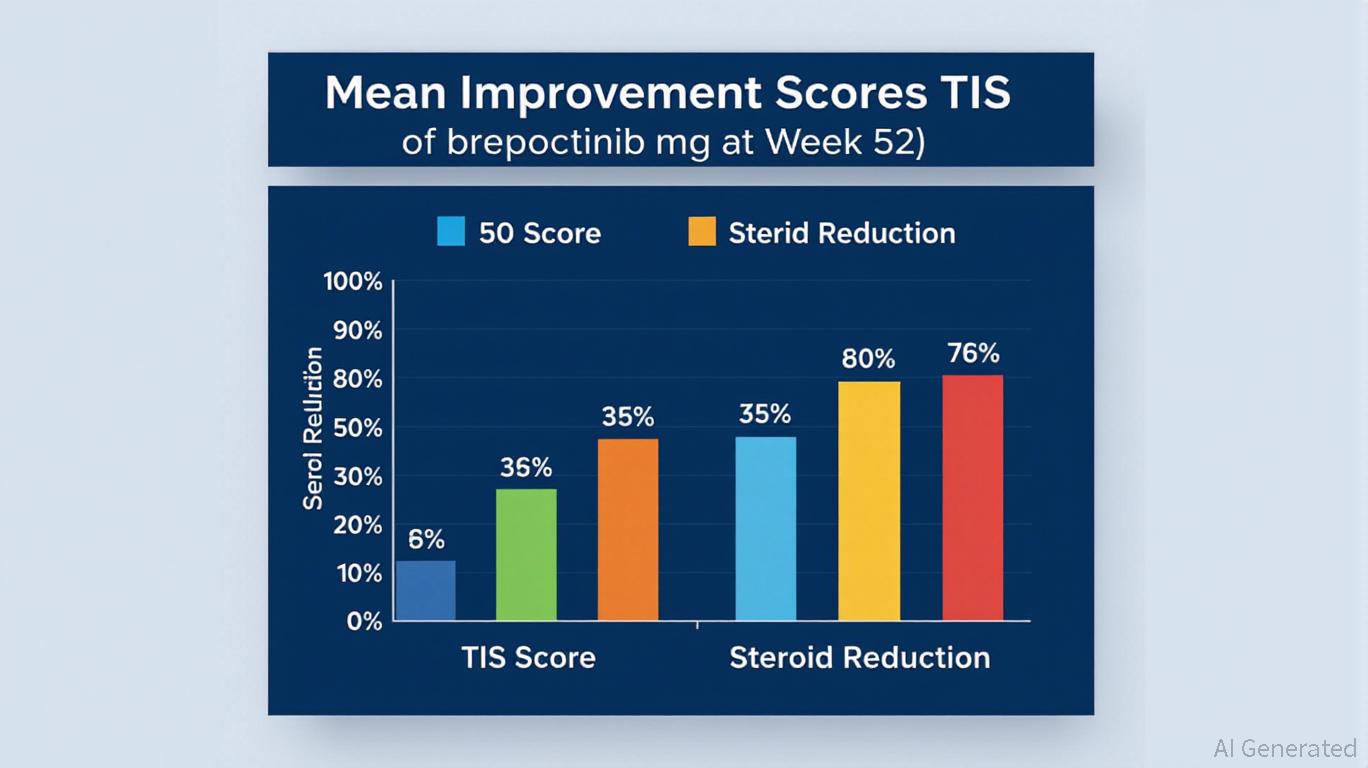
A drug licensed from Pfizer, brepocitinib, has demonstrated significant efficacy in a recent clinical trial for dermatomyositis, a rare inflammatory disease. The trial results indicate that patients experienced a reduction in symptoms as well as decreased reliance on steroid treatments. This promising data positions Roivant to submit the drug for FDA approval in the coming year, marking a critical step in addressing unmet medical needs in this patient population.
The context of this development is particularly relevant as dermatomyositis has limited treatment options, often leaving patients reliant on corticosteroids, which can have severe side effects. The successful trial results not only highlight the potential of brepocitinib as a novel therapeutic option but also underscore Roivant’s strategy of leveraging partnerships with established pharmaceutical companies like Pfizer to enhance its drug portfolio.
The implications of this advancement extend beyond patient care; they signal a growing interest in rare disease therapeutics within the pharmaceutical industry. As regulatory pathways evolve, successful outcomes like this could pave the way for increased investment and innovation in treatments for other rare and complex conditions.
Start your 7-day trial and see what the database can do →