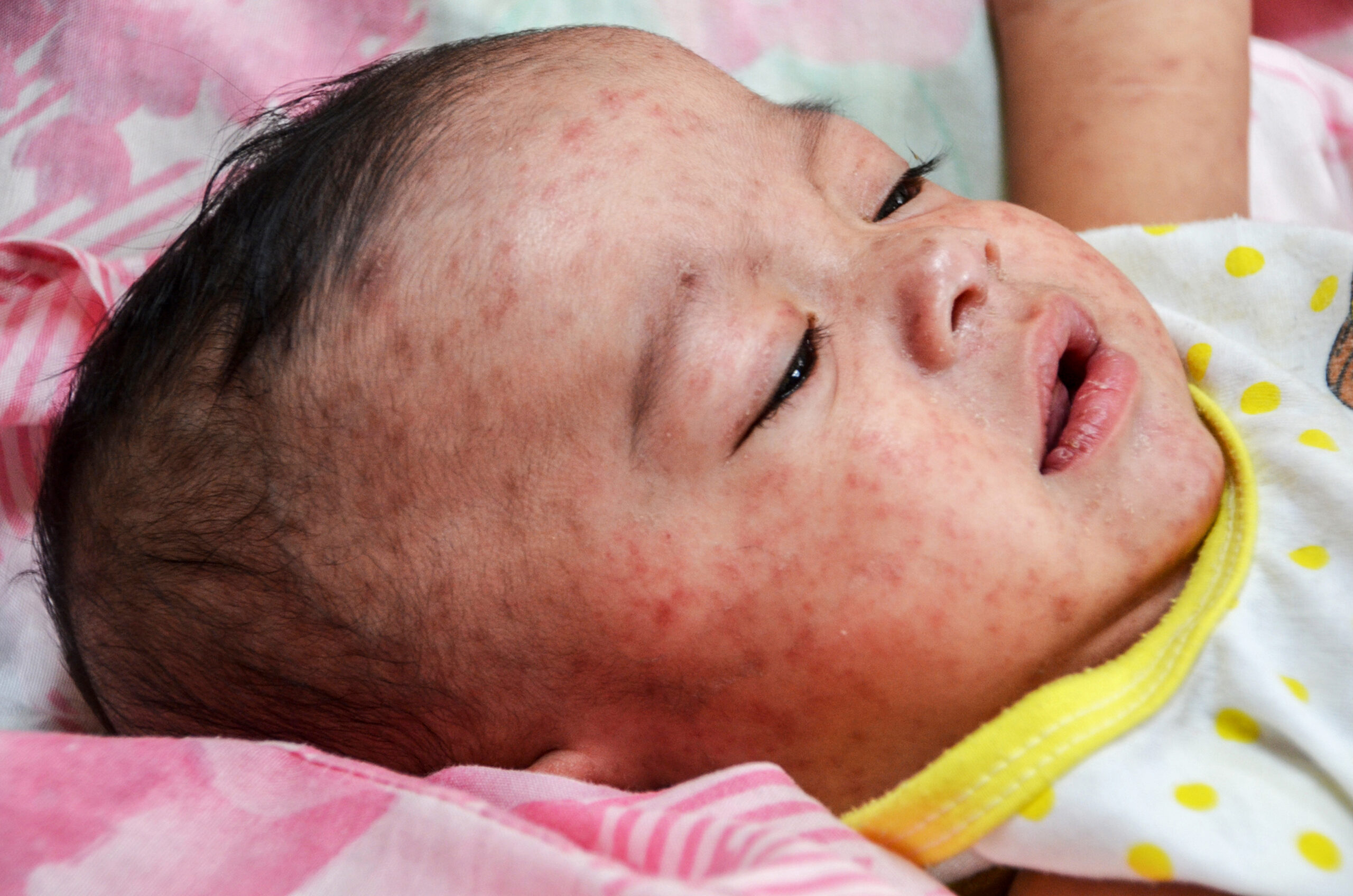
At this year’s AAP National Conference, a significant focus emerged on the concerns surrounding MMR (measles, mumps, and rubella) vaccines, as pediatricians gathered to discuss pressing health issues. The conference highlighted an increase in inquiries regarding the safety and efficacy of MMR vaccines, reflecting broader public apprehensions fueled by misinformation and vaccine hesitancy. This trend is particularly relevant as healthcare professionals strive to maintain high vaccination rates amidst rising cases of vaccine-preventable diseases.
The implications of these discussions are profound for pharmaceutical stakeholders, especially those involved in regulatory affairs, quality assurance, and supply chain management. As healthcare providers seek clarity and reassurance about vaccine protocols, there is a pressing need for pharmaceutical companies to bolster their communication strategies and ensure that accurate information is disseminated effectively. This not only safeguards public health but also fortifies the trust between healthcare providers and the pharmaceutical industry.
Start your 7-day trial and see what the database can do →