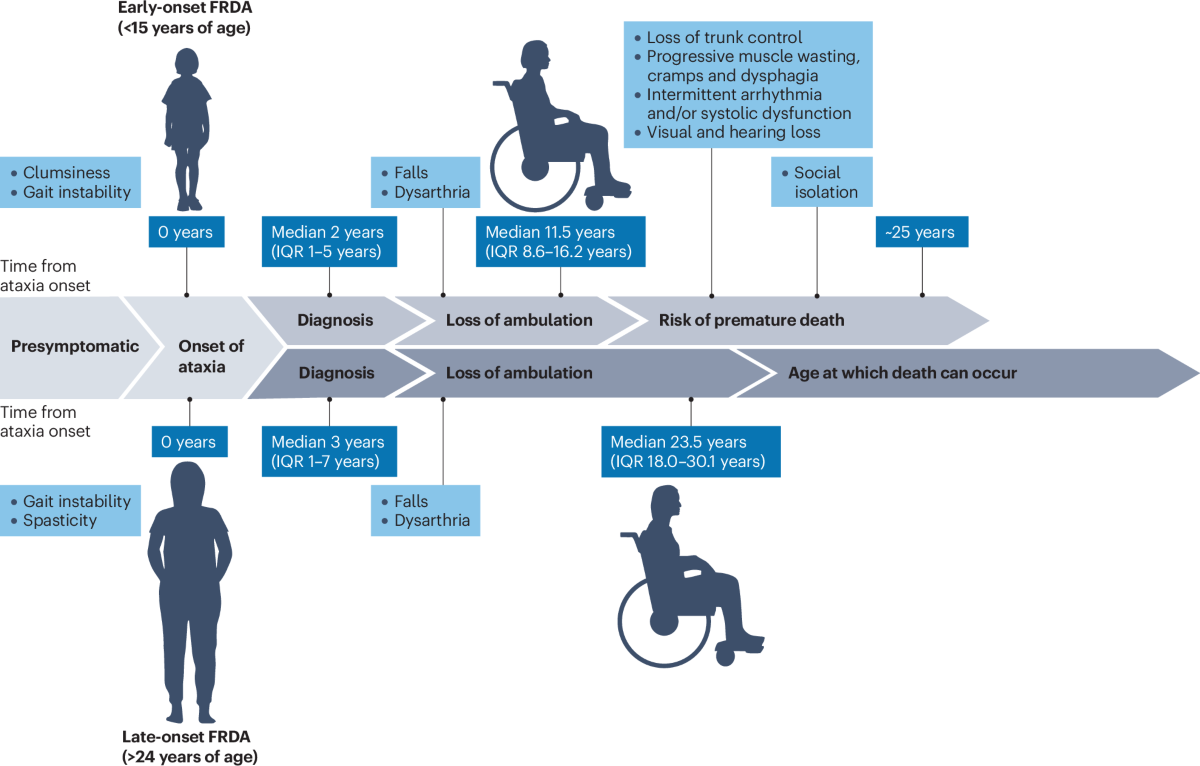
Larimar Therapeutics has reported significant challenges in the development of its experimental treatment for Friedreich’s ataxia, a rare neuromuscular disorder, as several patients have discontinued participation in the study due to allergic reactions. While initial data suggested potential efficacy, the safety concerns raised by these adverse events have cast a shadow over the drug’s future prospects.
This development is particularly concerning given the limited treatment options available for Friedreich’s ataxia, highlighting the delicate balance between therapeutic benefit and patient safety in clinical trials. The discontinuation of patients not only affects the integrity of the trial data but also raises questions about the formulation and administration of the drug.
The implications for Larimar are significant; the company may need to reassess its clinical strategy, potentially reformulating the drug or implementing more stringent safety protocols. For B2B professionals in the pharma sector, this case underscores the critical importance of robust safety evaluations and the challenges of bringing innovative therapies to market.
Use the database as your supply chain compass →