Chronic kidney disease (CKD) affects over 700 million people globally, with various contributing factors including genetics, environmental influences, and pre-existing medical conditions. Among the genetic risk factors, mutations in the apolipoprotein L1 (APOL1) gene, specifically the G1 and G2 variants, are particularly significant, especially in individuals of West African descent, where prevalence can reach 38% for carriers. Despite the established correlation between these mutations and increased CKD risk, the underlying mechanisms have remained largely unclear.
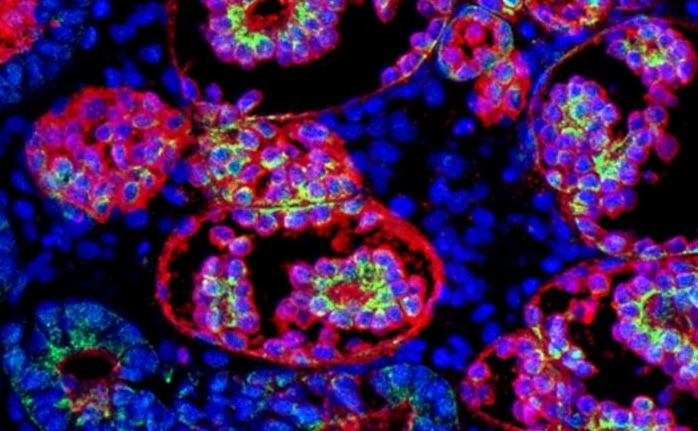
Recent research from the University of Leiden has leveraged kidney organoids derived from patient skin biopsies to investigate the impact of APOL1 mutations on CKD development. The study, published in Stem Cell Reports, utilized induced pluripotent stem cells (iPSCs) to create models that mimic human kidney disease. The findings revealed that APOL1 mutations lead to metabolic reprogramming in podocytes, characterized by decreased oxidative phosphorylation and increased glycolysis, suggesting mitochondrial dysfunction.
Moreover, under inflammatory conditions, such as exposure to interferon-gamma, these mutated podocytes exhibited severe mitochondrial impairment, potentially elucidating the accelerated progression of kidney disease following inflammatory events. This research not only enhances our understanding of APOL1-mediated kidney disease but also establishes a platform for developing therapeutic interventions aimed at restoring mitochondrial function and mitigating inflammatory responses, thus paving the way for future treatments of AMKD.
Open the full market picture for your next decision →