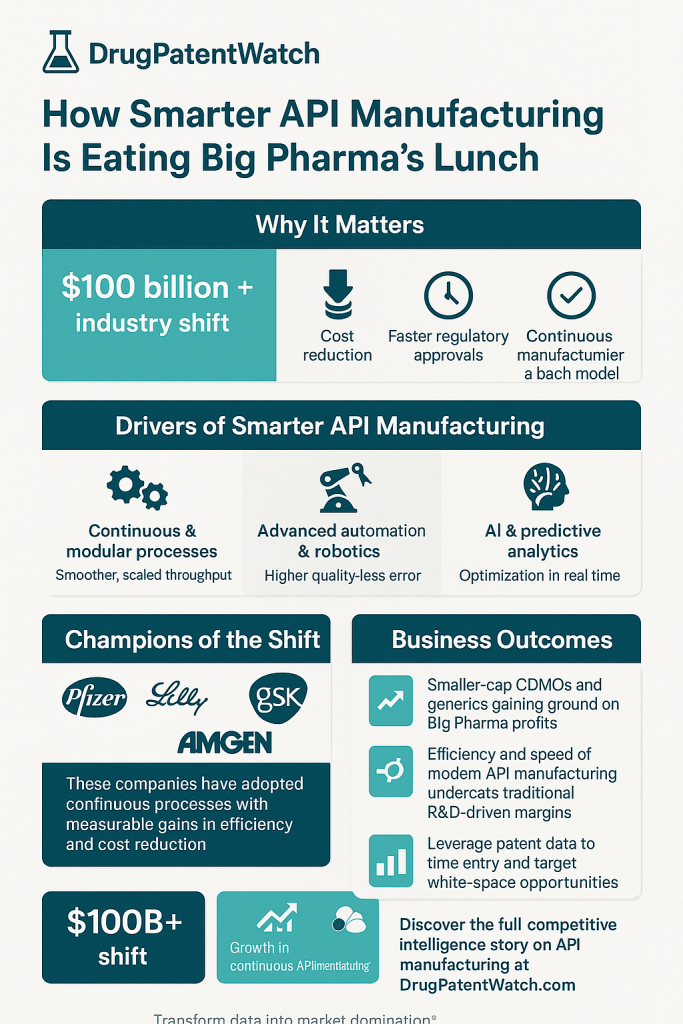
With the increasing demand for advanced therapies, the pharmaceutical industry is witnessing a significant shift towards advanced pharmaceutical manufacturing (APM) processes. These innovative manufacturing technologies aim to enhance drug quality, address shortages, and expedite time-to-market. Key technologies driving this transformation include continuous manufacturing, additive manufacturing, and digital integration. By applying these methods across various drug products and therapies, manufacturers can achieve improved process reliability and supply chain efficiency.
However, the transition to APM is not without challenges. High upfront costs, implementation barriers, and regulatory hurdles pose significant obstacles. To mitigate these issues, industry initiatives like the FDA’s Emerging Technology Program (ETP) and the Advanced Manufacturing Technologies Designation Program (AMTDP) are facilitating discussions between industry representatives and regulatory bodies. These initiatives aim to streamline the adoption of new technologies, particularly for essential medicines facing shortages.
Contract Development and Manufacturing Organizations (CDMOs) play a crucial role in supporting the adoption of APM by providing specialized expertise and innovative solutions. As the industry evolves, embracing APM technologies will be essential for manufacturers to remain competitive and responsive to market demands.
Get started today with Solo access →