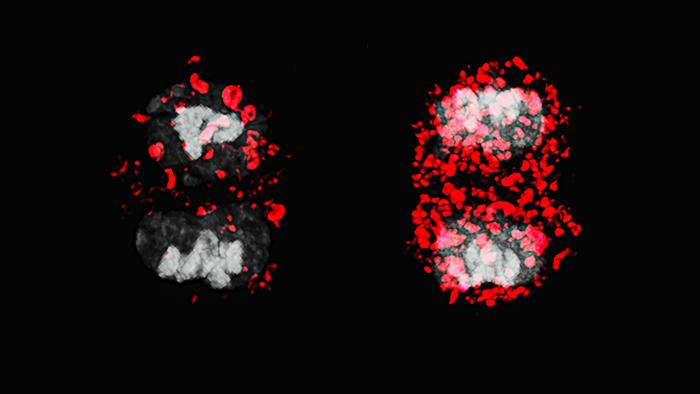
Researchers at the Max Delbrück Center and the University of Oxford have discovered that autophagy, a cellular process responsible for recycling components, is crucial for the asymmetric cell division (ACD) of T stem cells. Utilizing a specialized mouse model that allows for the sequential tagging of mitochondria, the study revealed that autophagy regulates the distribution of both healthy and damaged mitochondria between daughter T cells, ultimately influencing their fate.
Led by Dr. Mariana Borsa and Dr. Katja Simon, the findings published in Nature Cell Biology suggest that enhancing autophagy could lead to improved memory T cell production, potentially boosting vaccine responses in older adults. The research indicates that effective immune responses rely on the diversity generated within T cells during ACD, where one daughter cell becomes a long-lived memory T cell while the other becomes a short-lived effector T cell.
Importantly, the study provides the first causal evidence linking mitochondrial inheritance and T cell fate, highlighting the role of autophagy beyond mere cellular maintenance. Disruption of autophagy led to both daughter cells inheriting damaged mitochondria, thereby limiting their lifespan and functionality. By targeting autophagy, the researchers propose a novel therapeutic avenue for enhancing immune memory and vaccine efficacy, particularly in aging populations.
Start your 7-day trial and see what the database can do →