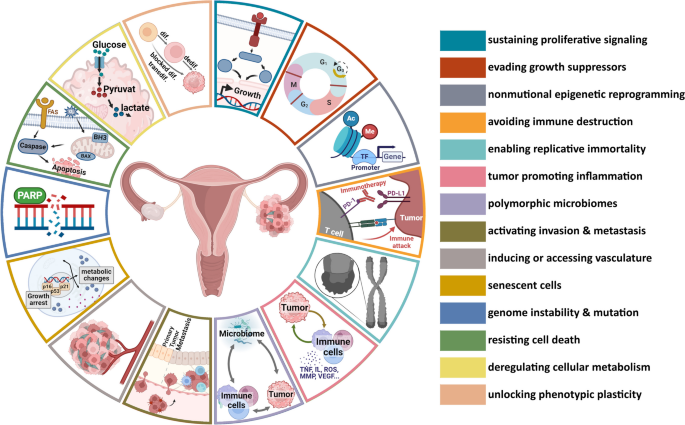
Avenzo Therapeutics has successfully raised $60 million in its latest funding round, aimed at advancing its portfolio of four investigational cancer therapies, which include two small molecules and two antibody-drug conjugates (ADCs) licensed from Chinese partners. This Series B financing brings the total capital raised by the San Diego-based biotech to $446 million, underscoring its aggressive strategy in the competitive oncology market.
The increasing trend of China-to-US licensing agreements highlights a significant shift in the pharmaceutical landscape, where innovative therapies developed in China are gaining traction in Western markets. Avenzo’s ability to secure substantial funding reflects investor confidence in its business model and the potential of its licensed assets to address unmet medical needs in cancer treatment.
As Avenzo continues to expand its pipeline, the implications for regulatory pathways and market access strategies will be critical. The success of these therapies could not only bolster Avenzo’s position in the oncology sector but also pave the way for similar collaborations between Chinese biotech firms and Western companies, ultimately enhancing the global therapeutic landscape.
Get started today with Solo access →