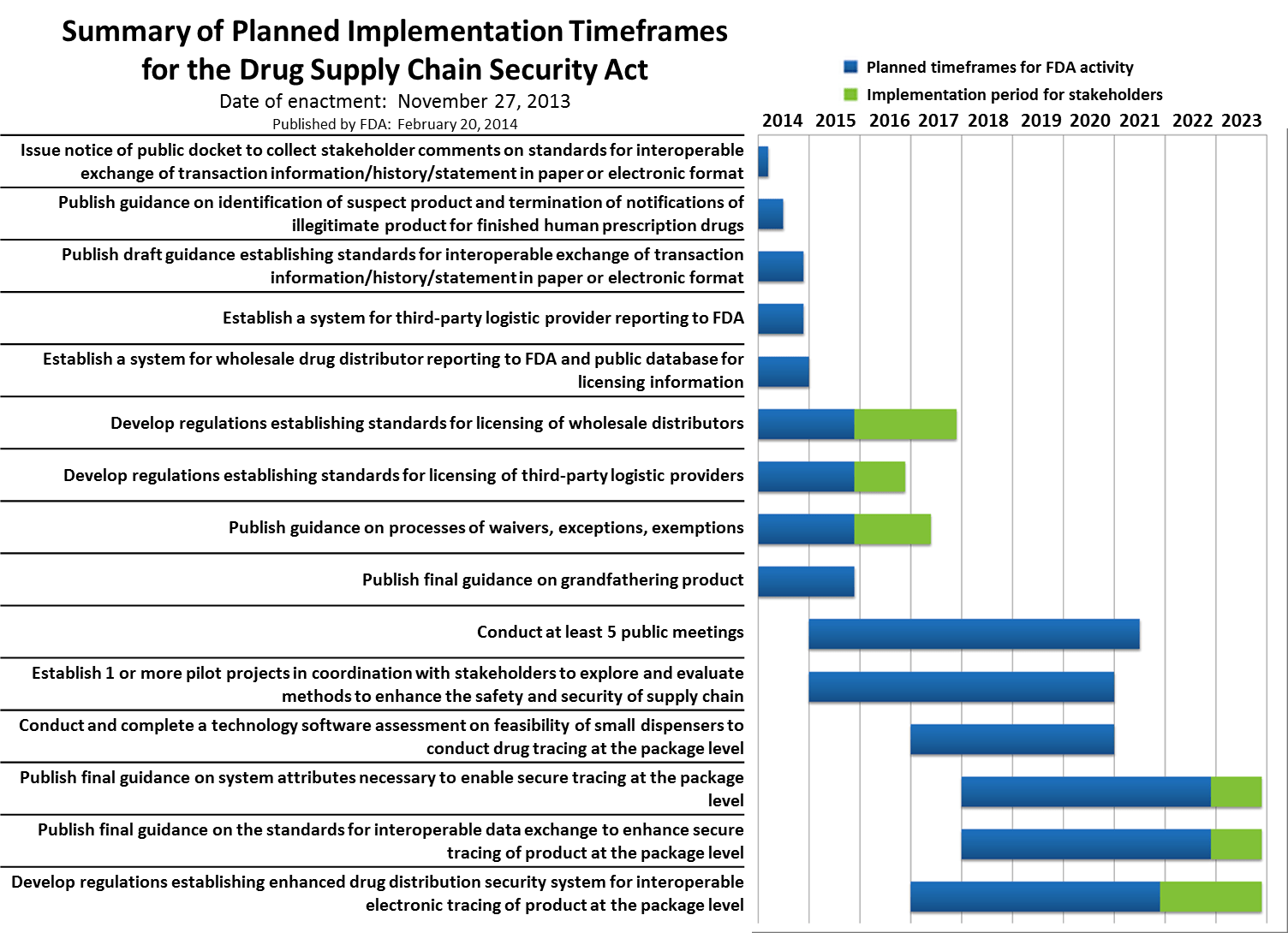
The BIOSECURE Act is on the brink of becoming law following its recent approval by Congress, a development that is expected to significantly impact US-China pharmaceutical relationships. This legislation aims to bolster the security of the pharmaceutical supply chain, addressing concerns over dependency on foreign manufacturers, particularly in China. As the global pandemic has underscored vulnerabilities in the supply chain, the BIOSECURE Act seeks to enhance domestic production capabilities and ensure that critical drugs and medical supplies are readily available in the United States.
The implications of this act are profound for B2B professionals across the pharmaceutical sector, including regulatory, quality assurance, and sourcing teams. Companies may need to reassess their supply chain strategies and compliance frameworks to align with the new regulations. Moreover, the act could lead to increased scrutiny of existing partnerships with Chinese manufacturers, prompting a reevaluation of sourcing practices and potential shifts in portfolio management to mitigate risks associated with international dependencies.
Open the full market picture for your next decision →