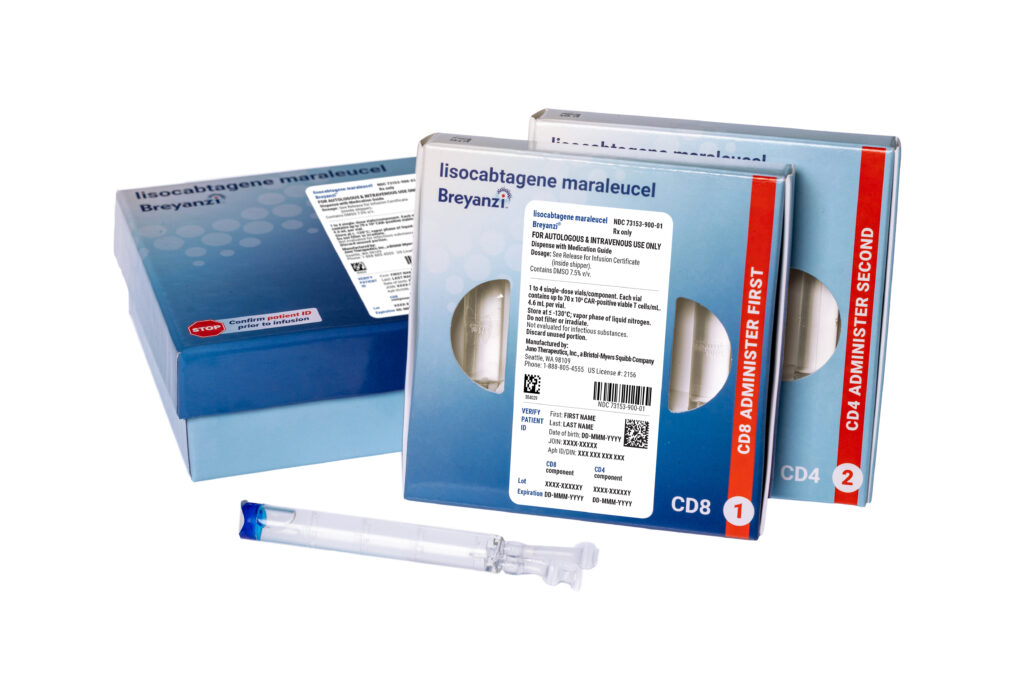
Bristol Myers Squibb’s CAR-T therapy Breyanzi has received approval for use in patients with marginal zone lymphoma, marking a significant milestone as it becomes the first CAR-T treatment sanctioned for this rare, slow-growing form of non-Hodgkin lymphoma. This approval, granted for patients who have undergone at least two prior therapies, underscores the evolving landscape of treatment options available for hematologic malignancies.
The expansion of Breyanzi’s indication reflects the growing recognition of CAR-T therapies in addressing unmet medical needs within niche patient populations. Marginal zone lymphoma, often characterized by its indolent nature, has historically presented challenges in management, particularly in later lines of treatment. With this approval, healthcare providers now have an innovative therapeutic option that may enhance patient outcomes and provide a new avenue for those who have exhausted other treatment modalities.
As the oncology market continues to evolve, the implications of this approval extend beyond patient care; it signals a shift in how biopharmaceutical companies are prioritizing research and development in specialized areas. The success of Breyanzi could pave the way for further advancements in CAR-T therapies, potentially leading to additional approvals for other lymphomas and hematological cancers.
Open the full market picture for your next decision →