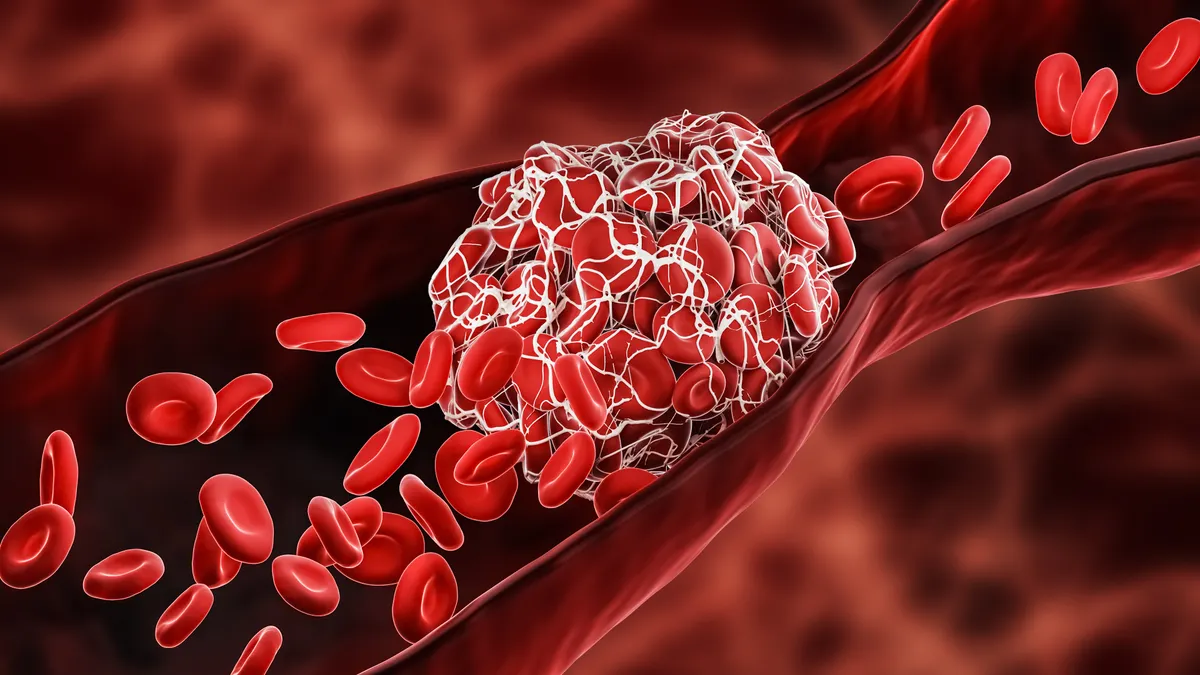
A closely watched next-generation blood thinner being developed by Bristol Myers Squibb and Johnson & Johnson failed in one of three late-stage trials. The drug, milvexian, is a selective factor XIa inhibitor designed to prevent harmful clotting in the blood. It is being tested in three Phase 3 trials, targeting acute coronary syndrome, atrial fibrillation, and prevention of secondary strokes. Bristol reported Friday that it is shutting down the first trial focused on acute coronary syndrome.
Acute coronary syndrome encompasses conditions where blood flow to the heart muscle is abruptly reduced, often due to a clot or heart attack. BMS indicated that an interim analysis revealed milvexian is unlikely to meet the primary endpoint of this trial. This development raises significant concerns regarding the drug’s efficacy and could impact the competitive landscape of anticoagulant therapies, particularly as the market continues to evolve with new entrants and innovative treatments.
Continue to STAT+ to read the full story…
Use the database as your supply chain compass →