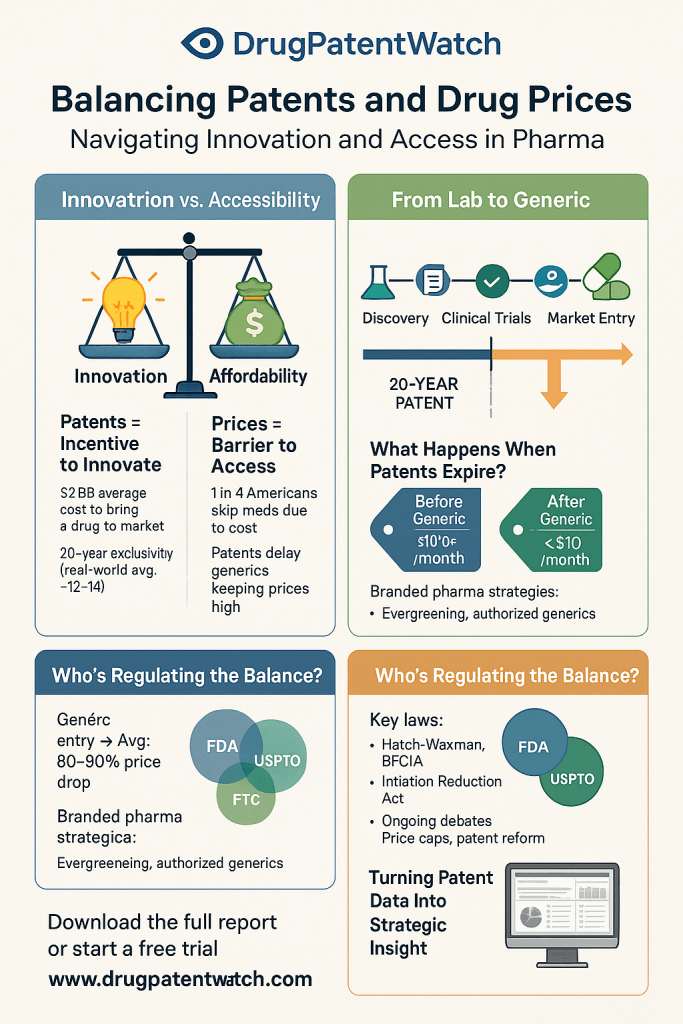
CDER researchers are advancing the use of deep learning, a subset of artificial intelligence, to better capture and analyze patient experiences with medical products. This innovative approach aims to facilitate the communication and retrieval of essential patient information, which is critical for regulatory assessments and product development.
The integration of deep learning technologies into the regulatory framework represents a significant shift in how patient feedback is utilized. By harnessing vast amounts of data, these methods can uncover nuanced insights that traditional analysis may overlook, thereby enhancing the understanding of patient needs and outcomes.
This development has profound implications for various stakeholders in the pharmaceutical industry, including regulatory professionals, quality assurance teams, and product developers. As patient-centric approaches gain traction, the ability to effectively analyze patient experiences will be crucial in informing regulatory decisions and optimizing product offerings.
Use the database as your supply chain compass →