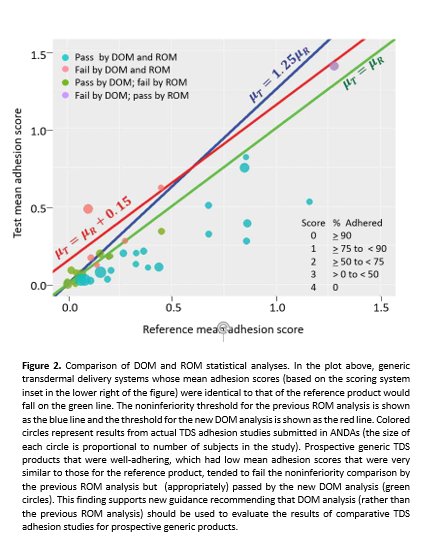
The Center for Drug Evaluation and Research (CDER) has introduced innovative statistical methodologies aimed at enhancing the assessment of adhesion for generic pharmaceutical products. This development is crucial as the pharmaceutical landscape increasingly demands rigorous evaluation techniques that keep pace with advancements in drug formulation and delivery technologies.
As the FDA continues to prioritize the safety and efficacy of medications, these new statistical measures are designed to provide more reliable data on how well generic products adhere to established standards. This shift not only underscores the importance of robust regulatory frameworks but also reflects a broader trend towards data-driven decision-making in drug approval processes.
The implications for industry stakeholders are significant; improved adhesion assessments could streamline the approval of generic products, potentially leading to greater market access and enhanced competition. As such, regulatory, QA/QC, and CMC professionals must stay informed about these developments to effectively navigate the evolving landscape of pharmaceutical regulations.
Use the database as your supply chain compass →