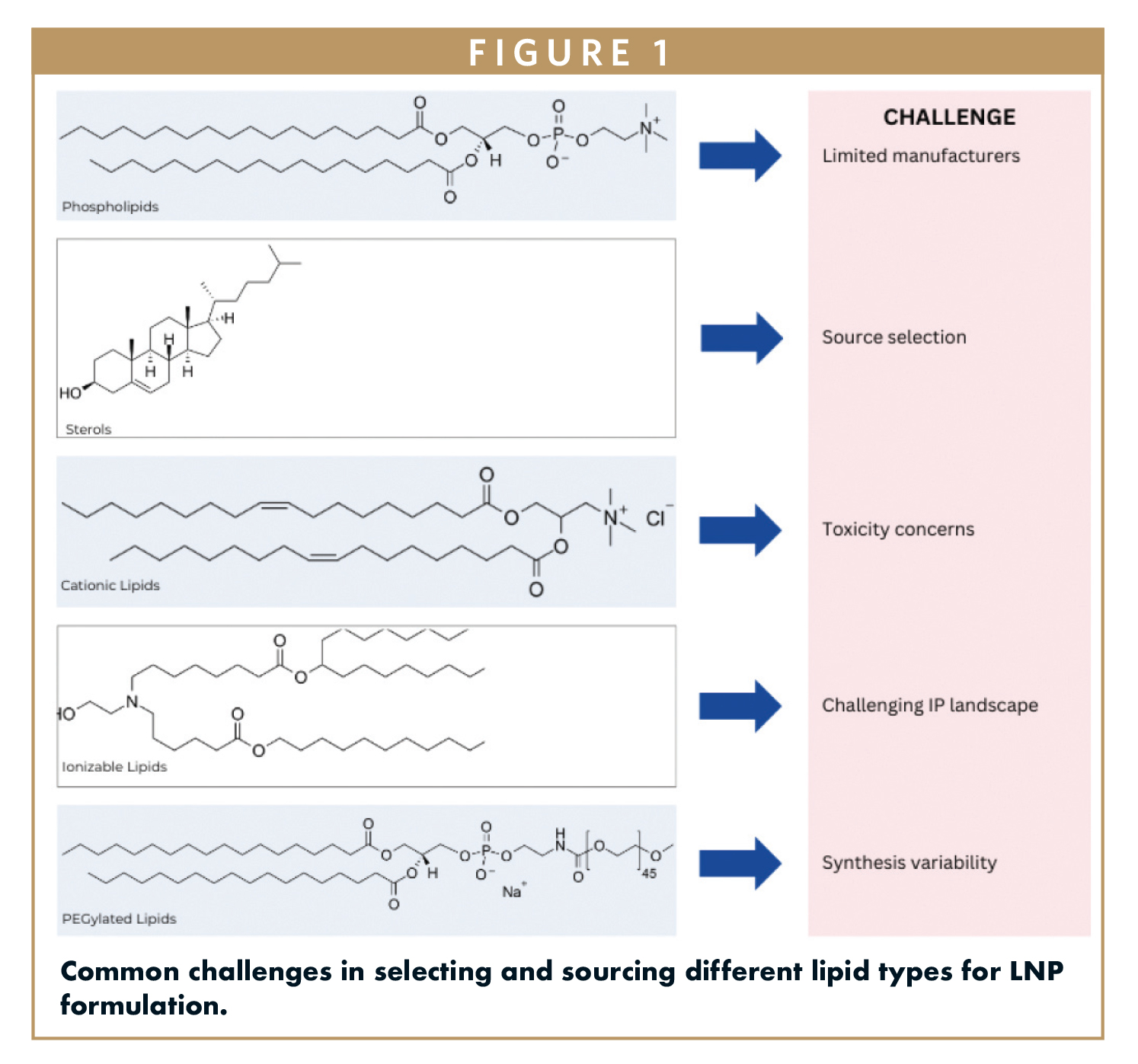
Cellares has announced multiple strategic partnerships aimed at advancing the capabilities of its Cell Q™ platform, a fully automated quality control testing system designed specifically for the commercial-scale throughput of cell therapy manufacturing. These collaborations, revealed ahead of the 10th Annual CAR-TCR Summit, will enable Cellares to showcase drug product release data generated by Cell Q, along with case studies that demonstrate the automation of critical processes such as system verification and vector copy number quantification.
Cell Q stands out as a modular and highly configurable QC platform that automates traditionally labor-intensive and error-prone assays. According to CEO Fabian Gerlinghaus, this innovative system can facilitate automated QC release testing for up to 6,000 cell therapy batches annually, thereby ensuring scalability that traditional manual QC infrastructures cannot match. The integration of technologies from partners like Tecan and Cytek Biosciences enhances the platform’s ability to streamline high-throughput sample preparation and real-time data tracking.
As regulatory scrutiny intensifies regarding data integrity and lifecycle management, Cell Q provides a robust framework for GMP compliance and digital traceability from the outset. This capability is particularly beneficial for early-stage developers looking to mitigate risks associated with tech transfer and expedite IND timelines. Furthermore, commercial developers can leverage Cell Q to reduce labor dependency and minimize batch failure risks, ultimately supporting global scalability. At the upcoming summit, Cellares’ senior director of automation will present insights on optimizing QC testing through automation, underscoring the platform’s potential to transform cell therapy manufacturing.
Use the database as your supply chain compass →