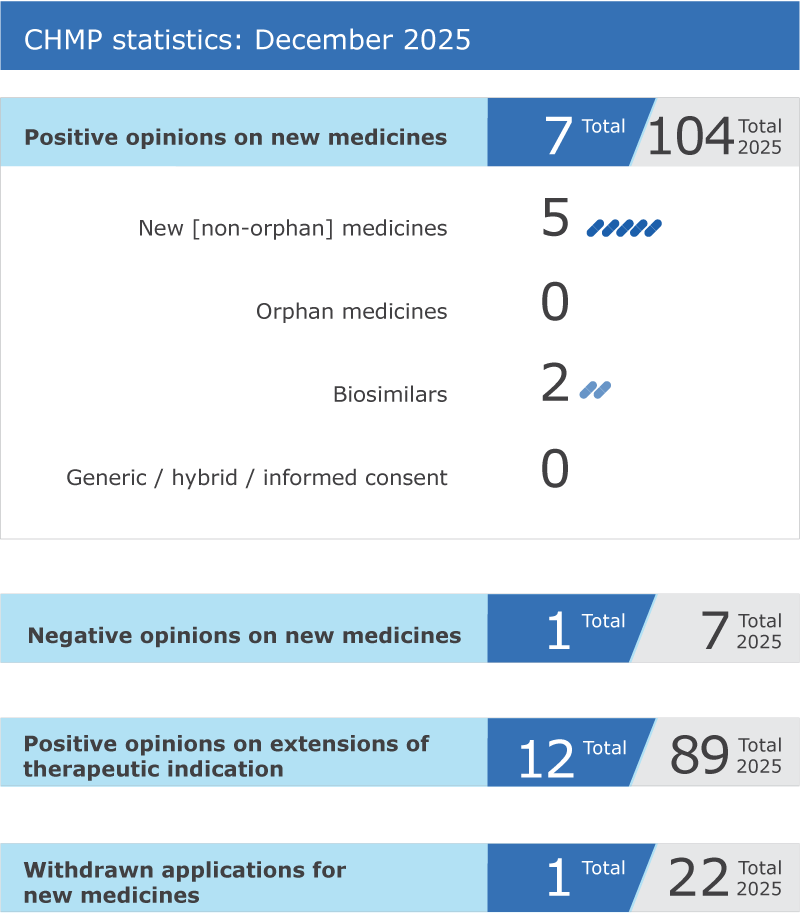
At its December 2025 meeting, the Committee for Medicinal Products for Human Use (CHMP) recommended seven new medicines for approval, marking a significant advancement in therapeutic options across various indications. This includes innovative treatments such as nogapendekin alfa for autoimmune conditions and aumolertinib for non-small cell lung cancer, highlighting the ongoing commitment to addressing unmet medical needs.
In addition to these new approvals, the committee also provided positive recommendations for several biosimilars, including golimumab and ranibizumab, which are crucial for enhancing treatment accessibility for chronic conditions like rheumatoid arthritis and age-related macular degeneration. However, the committee did issue a negative recommendation for blarcamesine, intended for Alzheimer’s disease, indicating ongoing challenges in neurodegenerative drug development.
The implications of these recommendations extend beyond immediate patient care; they signal a robust pipeline of pharmaceutical innovation that could reshape market dynamics and regulatory strategies in Europe. As companies prepare for potential market entry, the focus will likely shift to post-approval surveillance and ensuring compliance with evolving regulatory standards.
Get started today with Solo access →