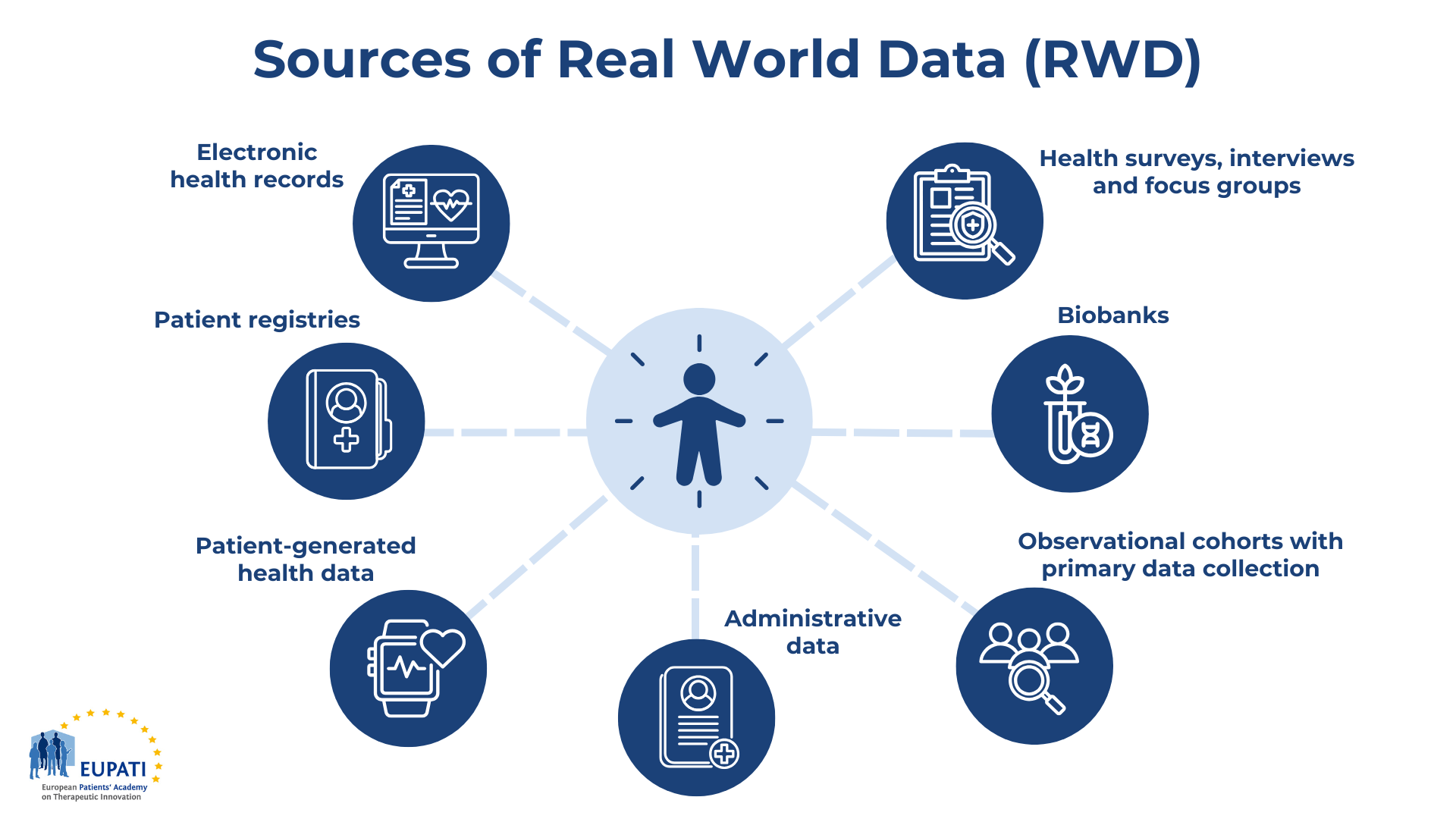
Families grappling with rare diseases often find that clinical trials, designed to test new therapies, are frequently inaccessible to them. This reality is underscored by the experience of families like mine, as I navigate the complexities of my granddaughter’s condition, Dravet syndrome, a severe form of epilepsy linked to genetic mutations.
Dravet syndrome, primarily caused by mutations in the SCN1A gene, presents significant challenges, including frequent seizures that persist despite the use of FDA-approved anti-seizure medications, many of which are prescribed off-label. The dilemma faced by my granddaughter’s parents illustrates a broader issue within the pharmaceutical industry: the tension between standard care and the need for innovative treatments. As they weigh the risks of ongoing seizures against the potential benefits of experimental therapies, the exclusionary nature of clinical trials becomes painfully evident, leaving many patients without viable options.
Start your 7-day trial and see what the database can do →