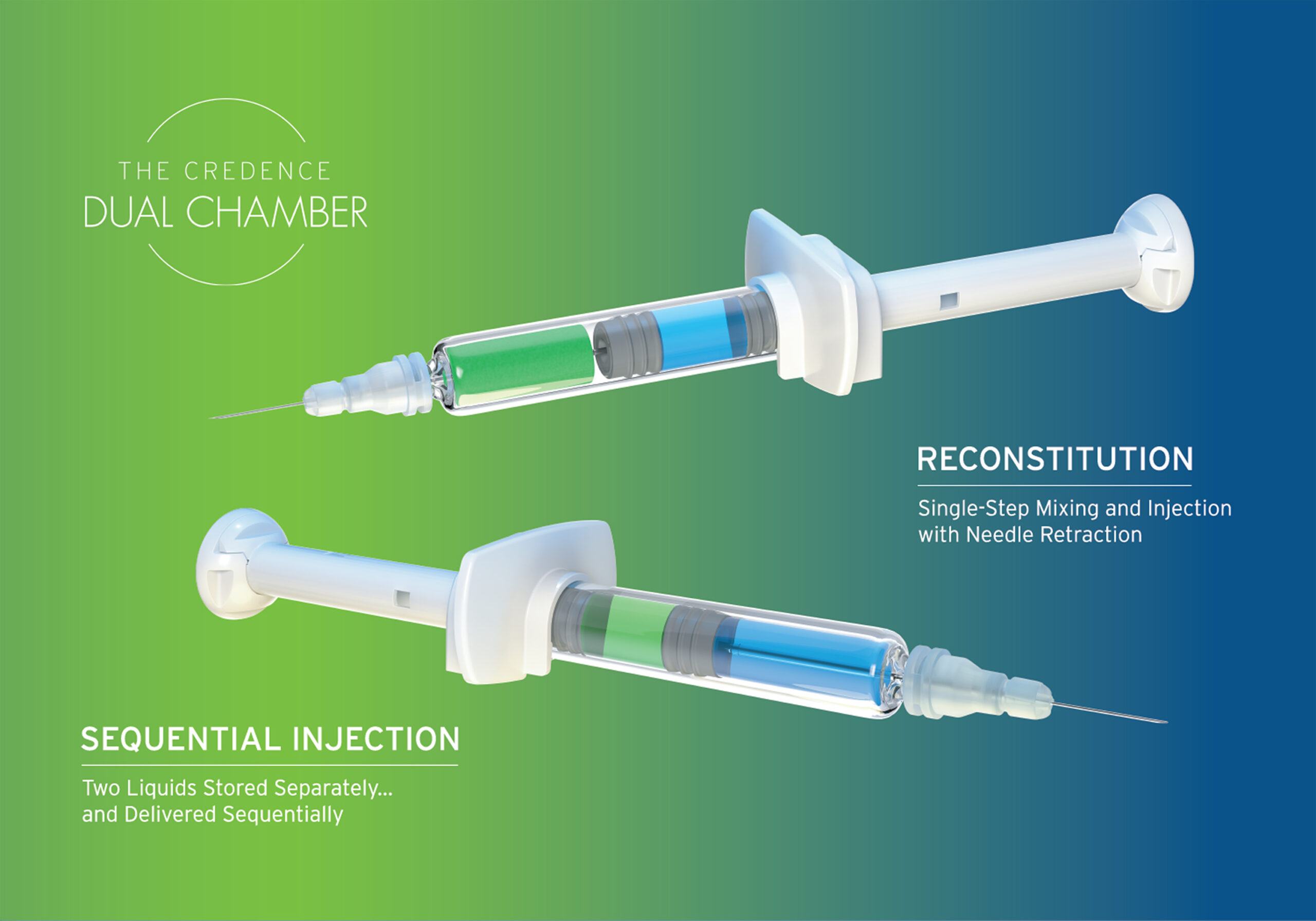
Credence MedSystems, Inc. and SMC Ltd. Pharma Services have signed a Memorandum of Understanding (MoU) to enhance dual chamber fill-finish capabilities for combination drug-device products. This collaboration aims to streamline the clinical and commercial-scale filling processes for Credence’s innovative Dual Chamber Syringe (DCS) platform, which is designed to meet the evolving needs of the pharmaceutical industry.
By leveraging SMC’s expertise in aseptic drug product development and regulatory support alongside Credence’s proprietary technology, the partnership is positioned to offer biopharma companies a comprehensive solution for advancing injectable therapies. The initial phase involves the installation of clinical-scale GMP fill-finish equipment at SMC’s facility in Charlotte, NC, which will support both Sequential Liquid-Liquid DCS and Lyo-Liquid Reconstitution DCS products.
This strategic alliance not only accelerates development timelines but also ensures adherence to the highest quality and compliance standards. As pharmaceutical companies increasingly seek advanced dual chamber therapies, this collaboration represents a significant step towards creating a robust manufacturing pathway that enhances the efficiency of bringing innovative therapies to market.
Start your 7-day trial and see what the database can do →