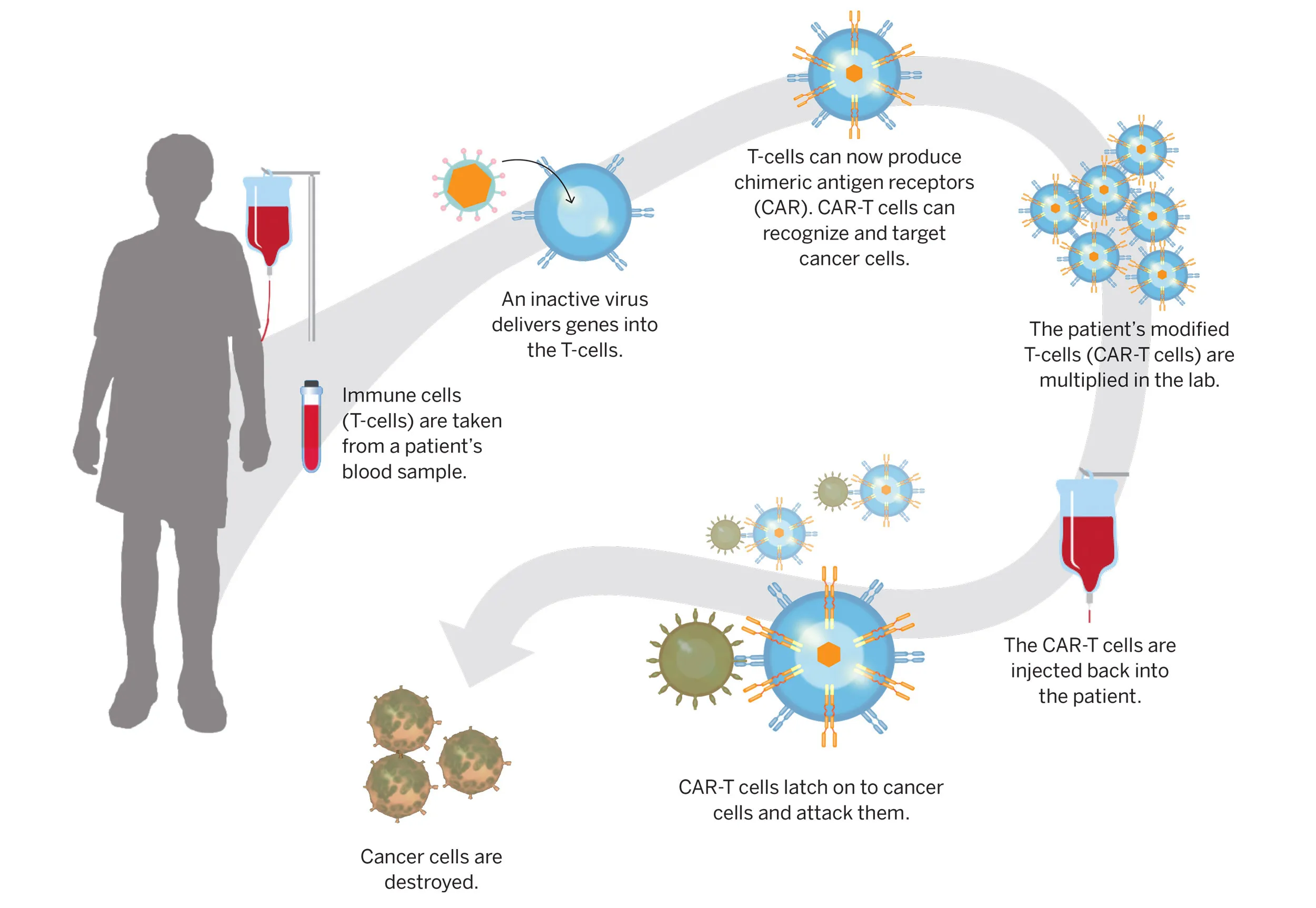
Clinical trials in cell and gene therapy (CAGT) are increasingly complex, driven by evolving regulatory landscapes and technological advancements. The intricacies of patient recruitment, site selection, and asset manufacturing are critical to the success of these trials. As the number of CAGT trial starts surged to 533 in 2024, up from just 171 in 2015, stakeholders must adeptly navigate the multifaceted landscape from discovery to commercialization. This heightened competition underscores the importance of early decision-making in trial design, patient engagement, and logistical planning.
With the rapid growth of CAGT R&D, sponsors face unique challenges, including identifying rare patient populations and ensuring robust supply chains for therapy delivery. Each decision made at the outset can have significant downstream implications for regulatory approval and market access. As emerging biopharmaceutical companies focus on a limited number of assets, their survival hinges on executing precise strategies that account for the complexities of CAGT. The promise of these therapies necessitates that stakeholders remain committed to overcoming these challenges, ensuring that innovative treatments reach patients who need them.