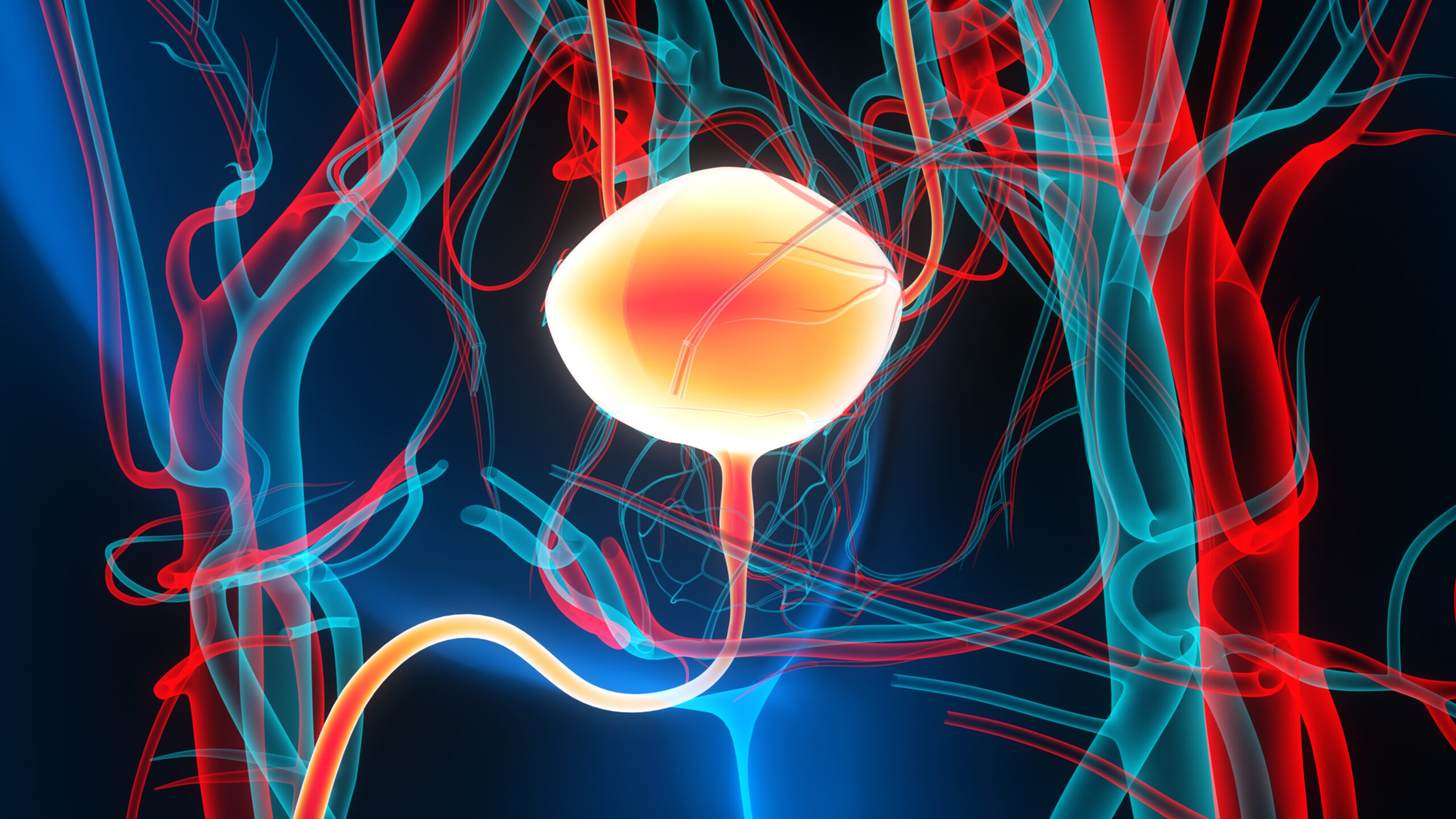
On September 9, 2025, the Food and Drug Administration (FDA) granted approval for the gemcitabine intravesical system, branded as Inlexzo, developed by Janssen Biotech, Inc. This approval specifically targets adults suffering from Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without accompanying papillary tumors.
This decision marks a significant advancement in the treatment landscape for NMIBC, particularly for patients who have not responded adequately to BCG therapy, which has long been the standard of care. The approval of Inlexzo not only expands therapeutic options but also underscores the ongoing need for innovative solutions in oncology, particularly in addressing treatment-resistant forms of cancer. As the healthcare community evaluates this new intervention, stakeholders across regulatory, quality assurance, and clinical development sectors will need to consider its implications for patient management and future research directions.
Use the database as your supply chain compass →