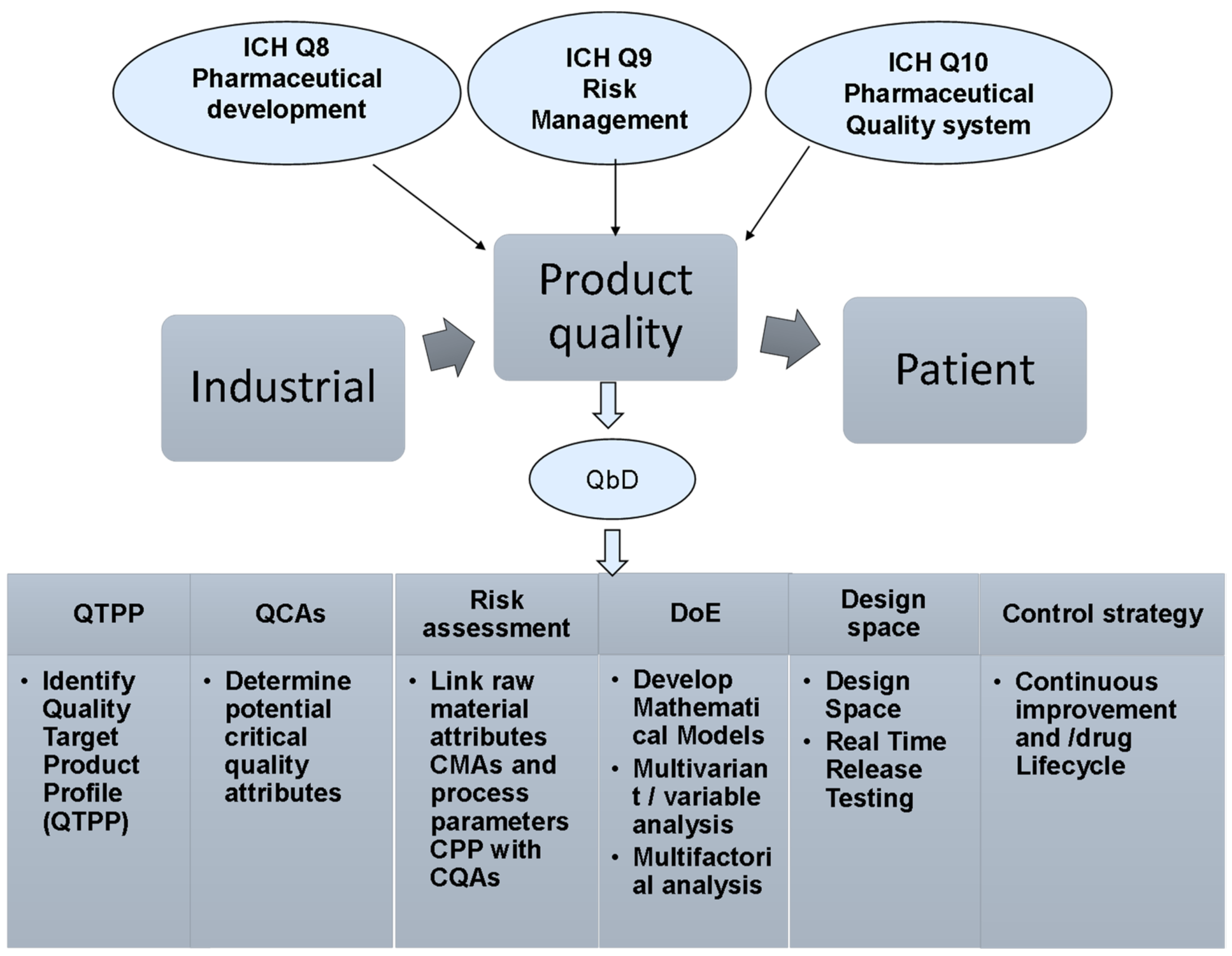
The FDA has developed and implemented analytical laboratory methods specifically designed to assess various quality attributes of drugs, which are critical in determining their safety for consumers. These methods are essential as they provide a framework for evaluating the integrity and reliability of pharmaceutical products in the market.
In the context of increasing scrutiny on drug quality and safety, these laboratory methods serve as a vital tool for regulatory professionals and quality assurance teams. They are part of a broader initiative to enhance pharmaceutical oversight and ensure that products meet stringent safety standards before reaching patients.
The implication of these developments is significant for B2B professionals in the pharmaceutical sector. As regulatory requirements evolve, companies must adapt their quality control processes to align with FDA standards, which may involve investing in new technologies and training for staff to maintain compliance and ensure product safety.
Get started today with Solo access →