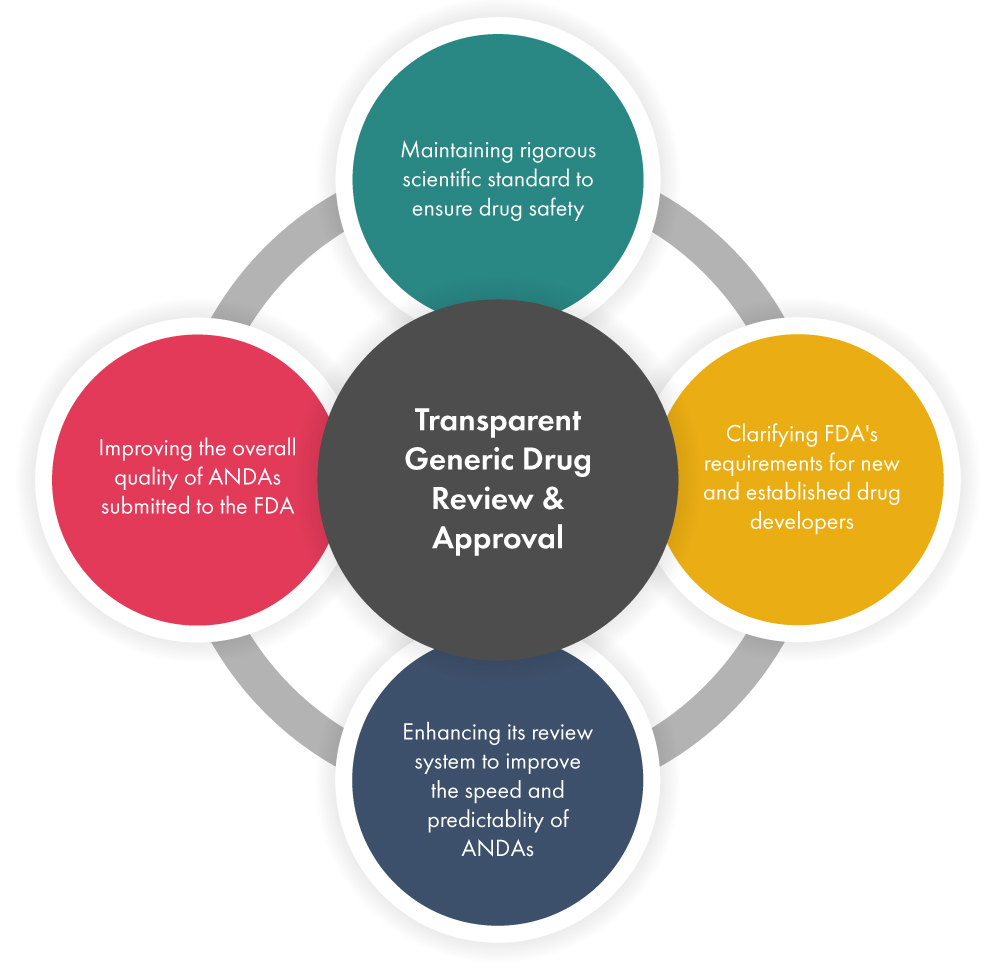
The FDA has unveiled its Drug Competition Action Plan, aimed at streamlining the development, review, and approval processes for generic drugs. This initiative is designed to clarify regulatory expectations for both new entrants and established drug developers, thereby fostering a more efficient pathway for bringing generics to market.
By providing clearer guidelines, the FDA is not only supporting prospective generic drug developers but also enhancing the overall quality of Abbreviated New Drug Applications (ANDAs) submitted for approval. This move is particularly significant in the context of rising drug costs and the ongoing need for affordable medication alternatives in the U.S. market.
The implications of this plan are profound, as it may lead to increased competition in the pharmaceutical sector, ultimately benefiting healthcare providers and patients alike by ensuring a more robust supply of generic medications.
Open the full market picture for your next decision →