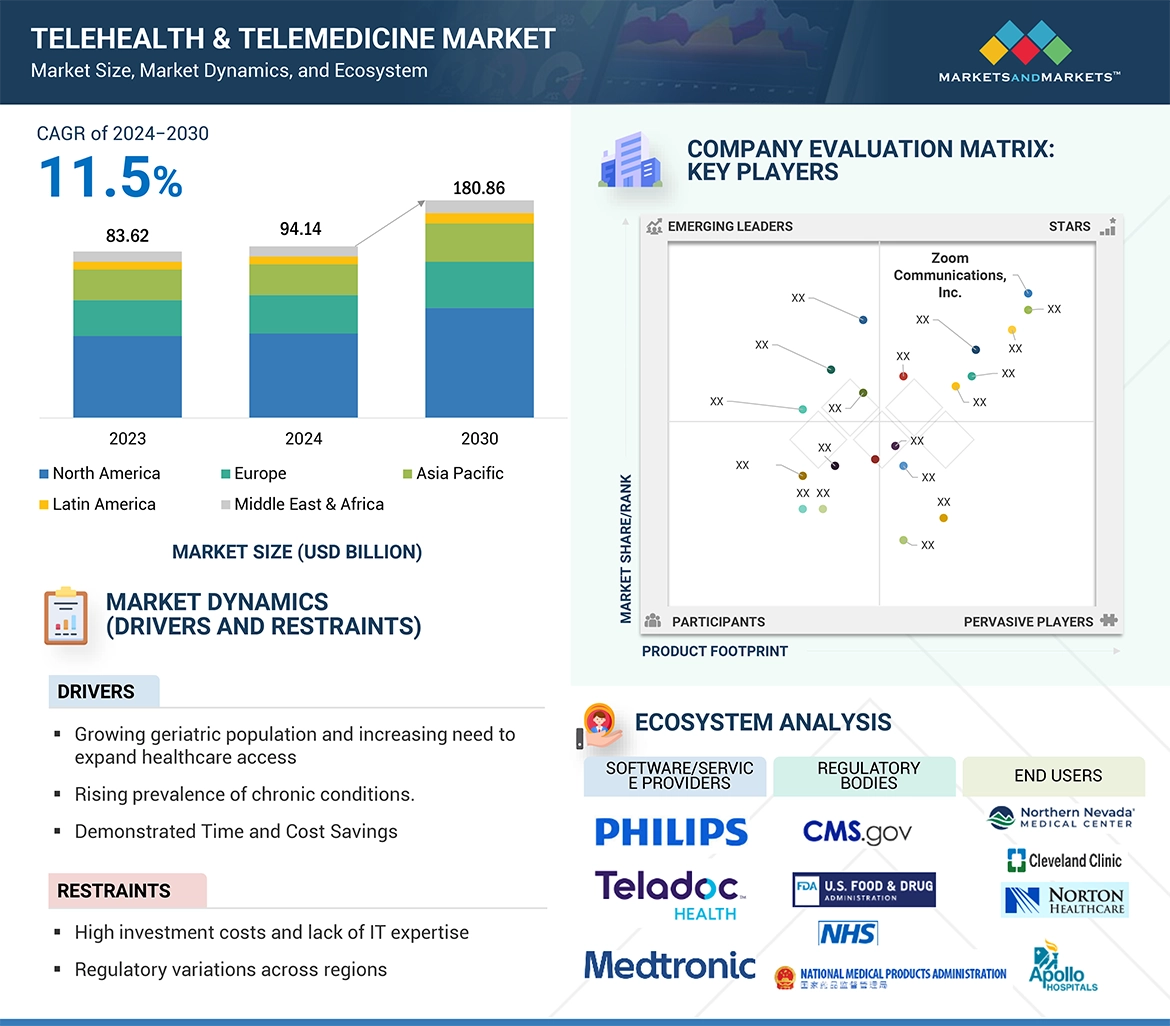
On July 2, 2025, the Food and Drug Administration granted accelerated approval to sunvozertinib (Zegfrovy, Dizal (Jiangsu) Pharmaceutical Co., Ltd.) for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) characterized by epidermal growth factor receptor (EGFR) exon 20 insertion mutations. This decision marks a significant advancement in the treatment landscape for a patient population that has historically faced limited therapeutic options.
The accelerated approval is based on clinical data demonstrating the efficacy of sunvozertinib in targeting specific genetic mutations, which is crucial as precision medicine continues to reshape oncology. With this approval, healthcare providers now have a new tool to address the unmet needs of patients suffering from this aggressive form of lung cancer, potentially improving outcomes and quality of life.
As the pharmaceutical industry responds to this regulatory milestone, stakeholders in regulatory affairs, quality assurance, and clinical development must prepare for the implications of this approval on market dynamics and patient access. The introduction of sunvozertinib could catalyze further research into targeted therapies, prompting a reevaluation of treatment protocols and the integration of genetic testing in clinical practice.
Start your 7-day trial and see what the database can do →