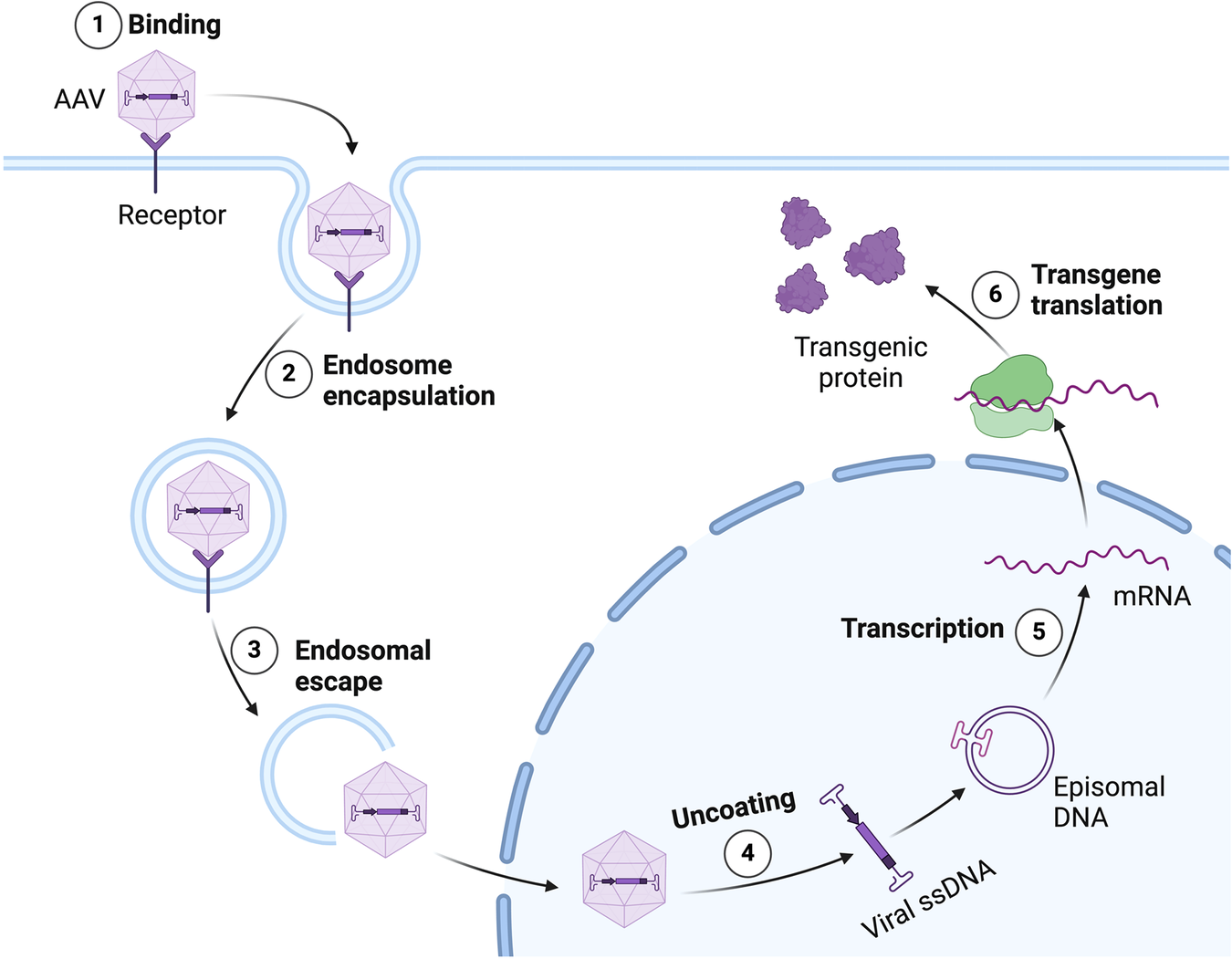
The U.S. Department of Health and Human Services and the Food and Drug Administration today announced sweeping reforms to rein in misleading direct-to-consumer pharmaceutical advertisements. This initiative aims to enhance transparency and ensure that consumers receive accurate information regarding prescription medications.
The context of this crackdown stems from growing concerns over the impact of misleading advertising on public health and patient decision-making. With the rise of direct-to-consumer marketing, the FDA recognizes the need to protect consumers from potentially harmful misinformation that could lead to inappropriate medication use. The reforms are expected to establish stricter guidelines and oversight mechanisms for pharmaceutical companies, thereby promoting ethical advertising practices.
The implications of these changes are significant for the pharmaceutical industry, particularly for regulatory, QA/QC, and marketing professionals. Companies will need to reassess their advertising strategies to comply with new regulations, potentially leading to increased operational costs and shifts in marketing approaches. As the FDA intensifies its scrutiny, firms must prioritize compliance to avoid penalties and maintain trust with healthcare providers and consumers alike.
Get started today with Solo access →