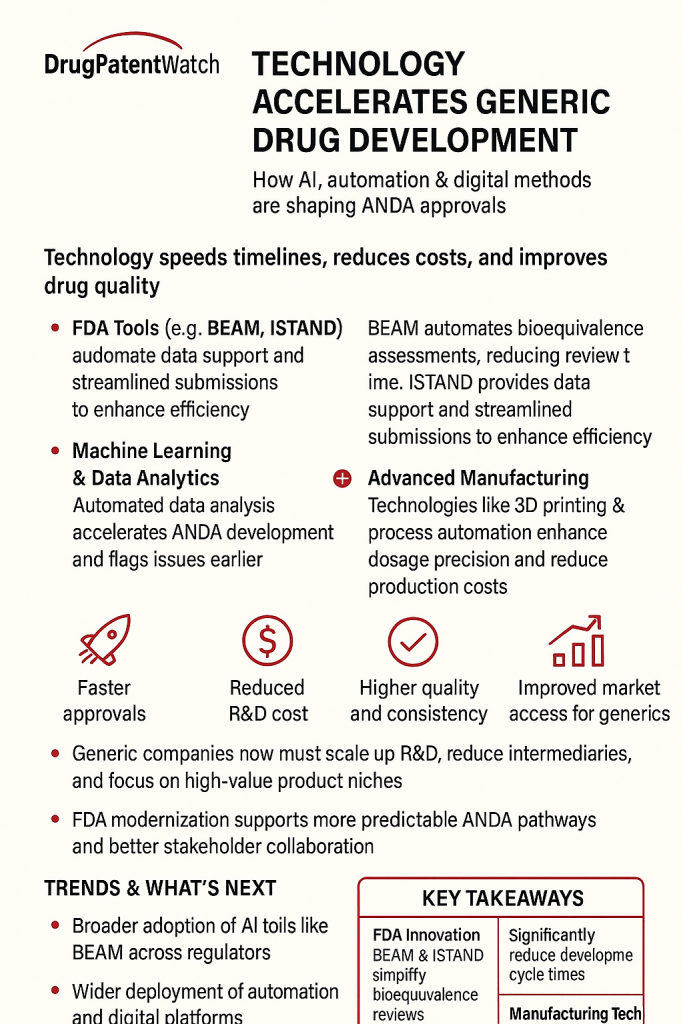
The FDA has released an updated chart detailing the approved biosimilar and interchangeable products, reflecting a significant advancement in the regulatory landscape. This update is crucial for pharmaceutical professionals in regulatory, QA/QC, CMC, sourcing, and portfolio management as it provides a comprehensive overview of available biosimilars.
Contextually, the approval of biosimilar products is designed to increase patient access to essential medications by broadening the range of treatment options. These products not only offer potential cost savings but also foster competition within the biopharmaceutical market, which can lead to improved patient outcomes.
The implications of this update are profound for stakeholders across the pharmaceutical industry. As biosimilars gain traction, companies must adapt their strategies to leverage these products effectively, ensuring compliance with evolving regulations while meeting the growing demand for affordable healthcare solutions.
Start your 7-day trial and see what the database can do →