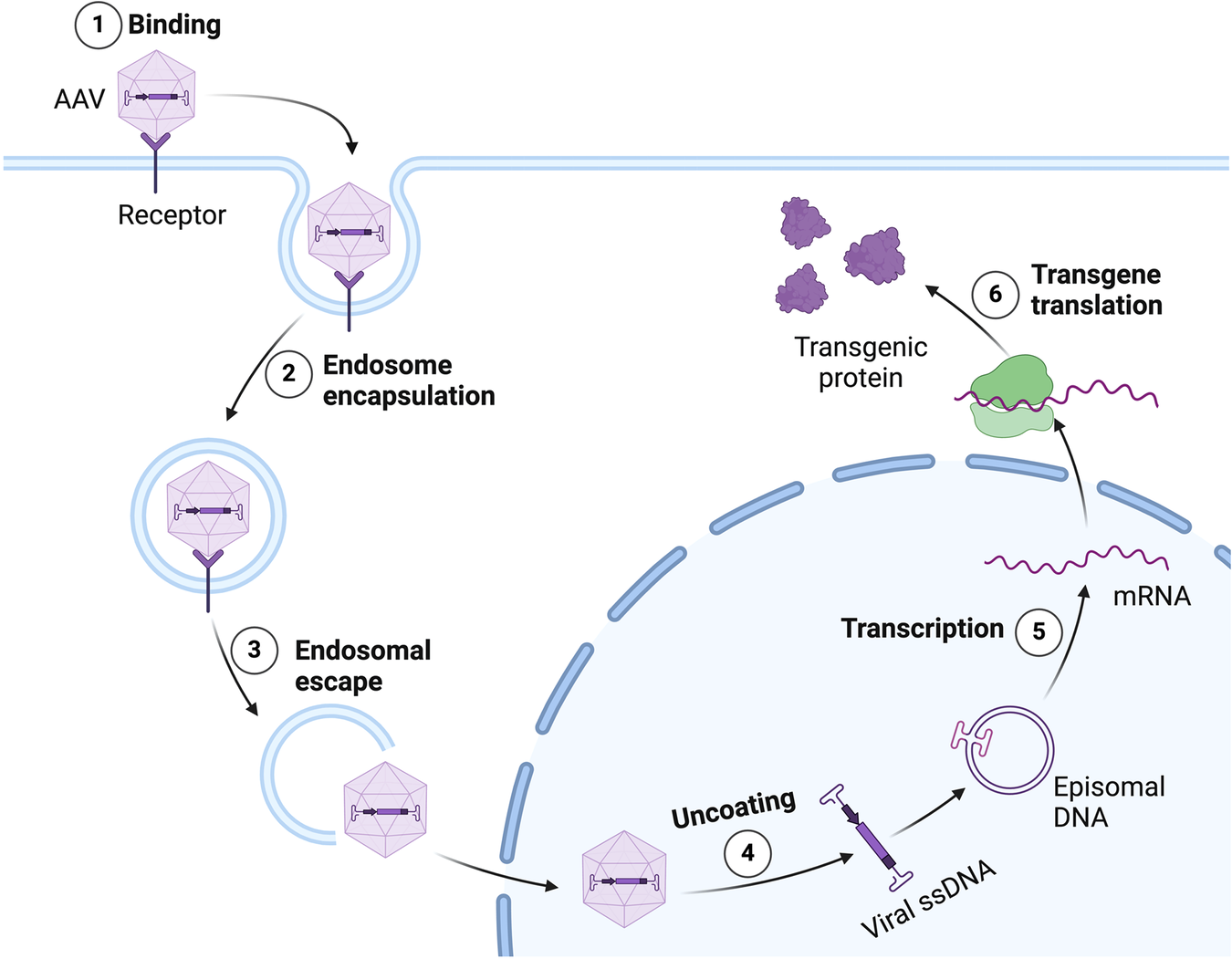
The FDA has revised the label for Sarepta’s Duchenne muscular dystrophy gene therapy, Elevidys, introducing a boxed safety warning and limiting its use to ambulatory patients. This significant regulatory action underscores the agency’s heightened scrutiny regarding the safety profile of gene therapies, particularly in vulnerable populations. The decision comes in light of ongoing discussions about the long-term effects and potential risks associated with such innovative treatments.
The implications of this label change are profound for both Sarepta and the broader gene therapy landscape. By restricting Elevidys to ambulatory patients, the FDA is signaling a cautious approach that may influence how other gene therapies are developed and marketed. Furthermore, the request for a postmarketing study indicates a commitment to ongoing safety monitoring, which could set a precedent for future gene therapy approvals and regulatory expectations.
Use the database as your supply chain compass →