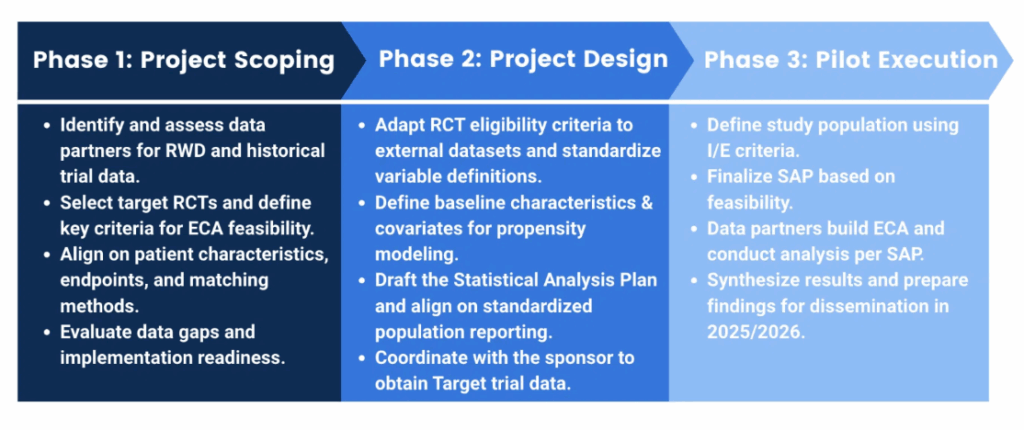
The US Food and Drug Administration’s Office of Therapeutic Products (OTP) held a public listening session on September 18, 2025, focusing on how prior knowledge can be utilized to accelerate the development and review processes for cell and gene therapy (CGT) products. This initiative signals a pivotal shift in regulatory strategy, emphasizing the need for developers and external partners to adapt to evolving guidelines and expectations.
During the session, industry experts discussed the importance of leveraging existing data across various domains, including chemistry, manufacturing, and controls (CMC), as well as clinical and nonclinical contexts. This approach aims to enhance efficiency, safety, and innovation in CGT development. For professionals in regulatory, QA/QC, and CMC roles, understanding these discussions is crucial for navigating the complexities of product development and compliance.
As the CGT landscape continues to evolve, the implications of the FDA’s insights will be significant. Stakeholders must consider how to effectively share valuable data, navigate partnerships, and integrate knowledge across disciplines to strengthen their development strategies and risk assessments. This proactive engagement with regulatory expectations will be essential for the successful commercialization of innovative therapies.
Get started today with Solo access →