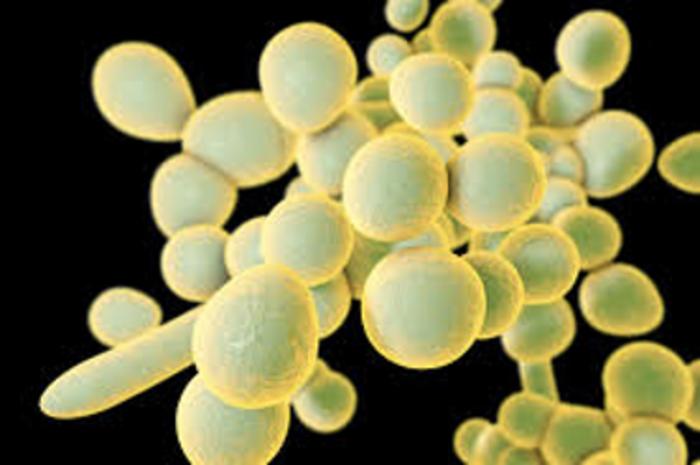
Candida species are increasingly recognized as significant contributors to invasive fungal diseases, particularly among hospitalized and immunocompromised individuals. The existing treatment landscape is constrained by a limited array of antifungal medications and vaccines, exacerbated by rising resistance rates, notably in Candida auris and Candida albicans. This scenario underscores an urgent need for innovative therapeutic approaches to combat Candida infections.
The Lundquist Institute (TLI) and Vitalex Biosciences have announced advancements in the development of their second-generation fungal vaccine candidate, VXV-01, which is set to progress into Phase I clinical trials. This dual-antigen vaccine aims to stimulate strong immune responses against critical opportunistic pathogens associated with hospital settings.
With a substantial contract from the National Institutes of Health (NIH/NIAID) providing up to $40 million in non-dilutive funding, the program is now equipped to commence manufacturing and preparatory activities for the upcoming trials. Dr. Ashraf Ibrahim, principal investigator at TLI and CEO of Vitalex, emphasized the significance of this funding as a transformative step towards realizing the vaccine’s potential. This initiative not only marks a key milestone for TLI’s vaccine research program but also reflects the organization’s long-standing commitment to addressing pressing infectious disease challenges.
Vitalex, a start-up dedicated to advancing TLI’s innovations, holds exclusive licensing rights for VXV-01. The lab’s focus includes developing vaccines targeting multidrug-resistant strains of Candida and Gram-negative bacteria, while Appili Therapeutics continues to expand its portfolio of anti-infective solutions.
Use the database as your supply chain compass →