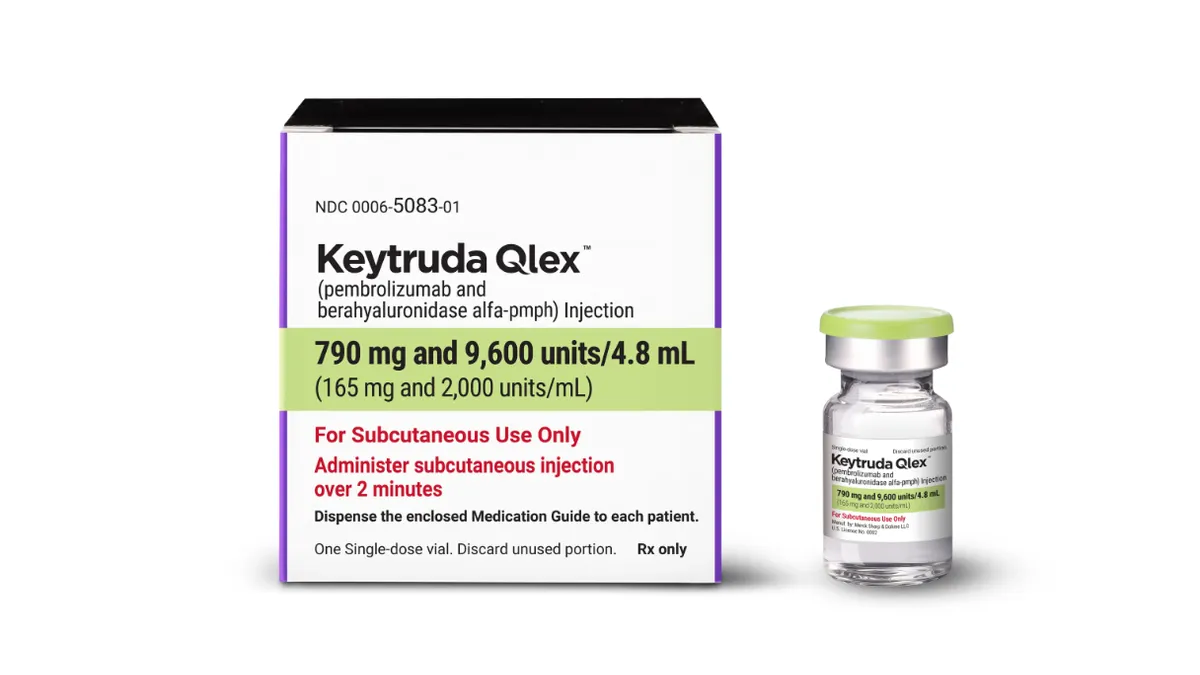
Wall Street analysts expect the subcutaneous version of Keytruda, which just launched this week, to help soften the blow when the original loses patent protection later this decade. This development comes at a critical time for Merck, as approximately half of its sales are tied to Keytruda, a cornerstone of its oncology portfolio.
The introduction of the subcutaneous formulation is not merely a product launch; it represents a strategic pivot to maintain market share in an increasingly competitive landscape. As patent expirations loom, the pressure mounts on Merck to innovate and retain its foothold in the immuno-oncology sector.
If successful, the new formulation could provide a much-needed revenue stream, mitigating the anticipated decline in sales from the original Keytruda. This scenario underscores the importance of adaptive strategies in pharmaceutical development, particularly in the face of patent cliffs and evolving treatment paradigms.
Get started today with Solo access →