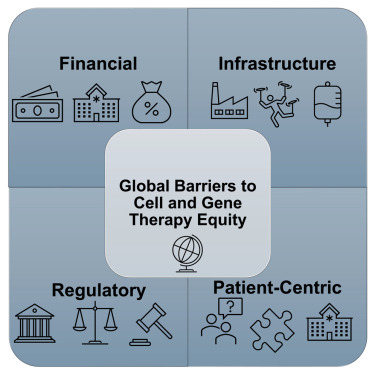
Recent discussions by industry experts Zoey Becker and Glenn Pierce, M.D., Ph.D., reveal that hemophilia gene therapies are struggling with substantial barriers to market adoption. These challenges stem from a combination of regulatory hurdles, high treatment costs, and the complexity of patient selection criteria. As the market for these innovative therapies expands, the implications for stakeholders in the pharmaceutical sector are profound.
The context surrounding these barriers highlights a critical juncture for gene therapies in hemophilia treatment. With numerous therapies emerging, the potential for transformative patient outcomes is overshadowed by the realities of reimbursement issues and logistical hurdles in patient access. This scenario raises concerns about which patient populations may be excluded from benefiting from these advancements.
For regulatory, QA/QC, CMC, sourcing, and portfolio management professionals, understanding these dynamics is essential. The ongoing evolution of hemophilia gene therapies not only reflects the complexities of modern pharmaceutical development but also underscores the need for strategic planning and collaboration across sectors to ensure equitable access for all patients.
Open the full market picture for your next decision →