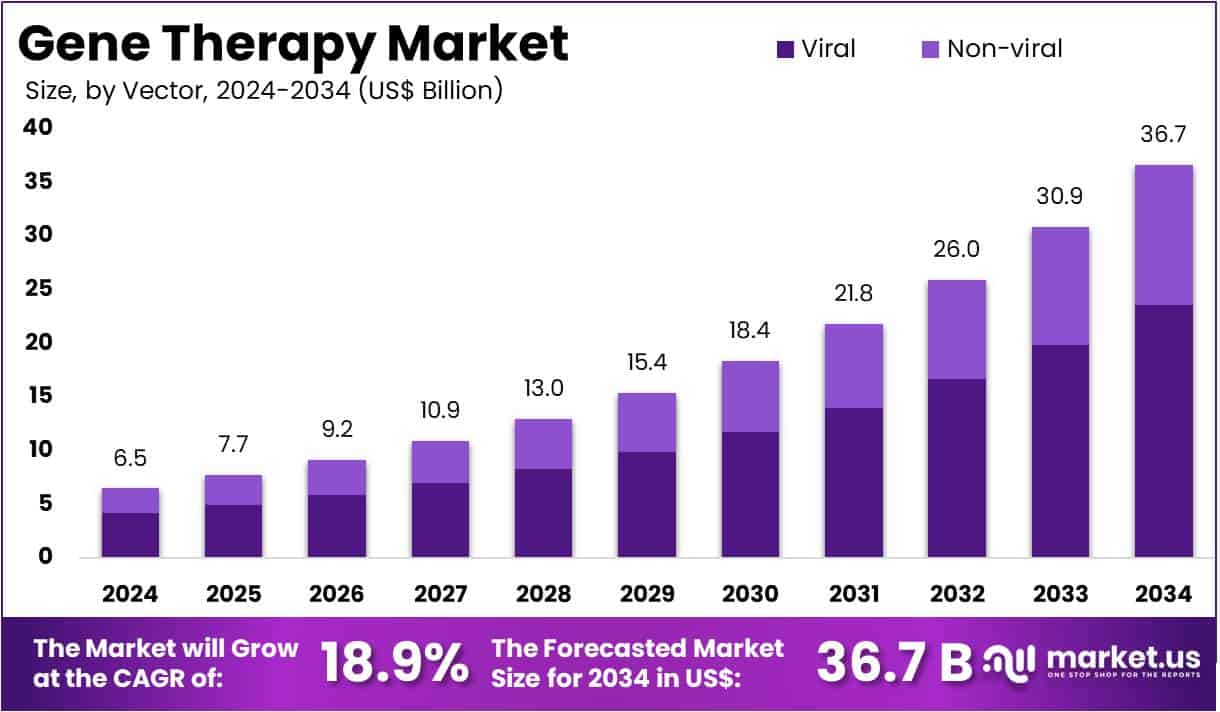
The business case for hemophilia gene therapy still isn’t adding up due to persistent market barriers. Despite the rapid advancements in gene therapy technologies and the promise they hold for treating hemophilia, recent market analyses indicate that uptake remains sluggish. Key players in the sector, including BioMarin, CSL, and Pfizer, are grappling with regulatory hurdles, pricing pressures, and reimbursement challenges that hinder widespread adoption.
This situation reflects a broader trend in the pharmaceutical industry where innovative therapies often struggle to gain traction in a complex market landscape. The implications for stakeholders are significant; regulatory professionals must navigate evolving guidelines, while QA/QC teams face increased scrutiny over product efficacy and safety. For sourcing and portfolio managers, the challenge lies in balancing investment in cutting-edge therapies with the realities of market acceptance and financial viability.
Open the full market picture for your next decision →