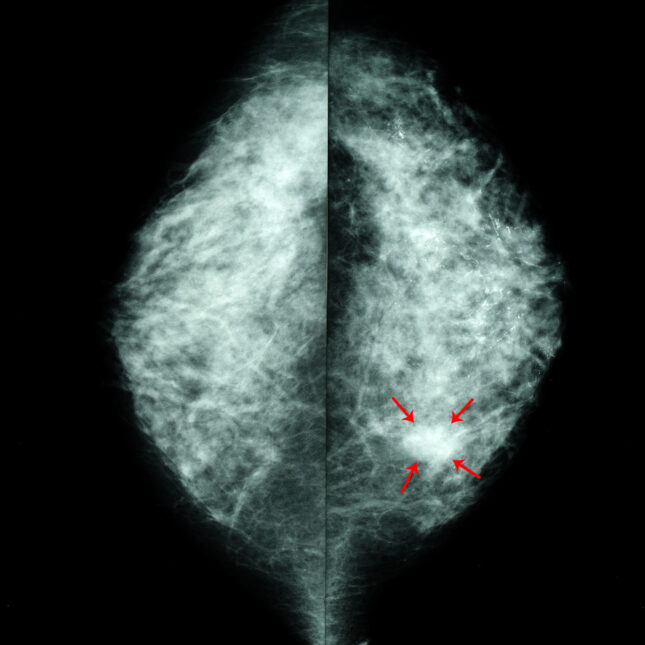
Researchers at medical centers across the United States have initiated the first large-scale randomized controlled trial assessing the efficacy of artificial intelligence in screening mammography. This $16 million, multi-site study represents a critical advancement in establishing a robust evidence base for AI technologies already utilized in millions of mammograms annually.
While AI-assisted mammograms are increasingly integrated into certain breast imaging practices, clinicians face challenges in fully understanding their implications for patient outcomes. Preliminary trials in Europe suggest that AI tools may enhance cancer detection rates; however, these findings are not directly applicable to the U.S. context, where mammograms are typically interpreted by a single radiologist. This discrepancy has led to skepticism among imaging specialists regarding the technology’s effectiveness and optimal application.
Diana Miglioretti, co-lead of the U.S. Breast Cancer Surveillance Consortium and head of the trial’s data coordinating center at UC Davis Health, emphasized that despite the push from companies to adopt AI, most existing evidence is derived from artificial settings that do not translate to real-world patient impacts.
Start your 7-day trial and see what the database can do →