As pharmaceutical and biotech companies strive to deliver innovative therapies, the choice of drug formulation plays a crucial role in the success of these endeavors. The industry faces significant challenges, particularly concerning the low bioavailability of many compounds, which can hinder drug progression beyond preclinical stages. Despite this, many organizations continue to favor traditional oral solid dosage (OSD) forms like tablets and capsules, primarily due to established manufacturing processes and regulatory pathways.
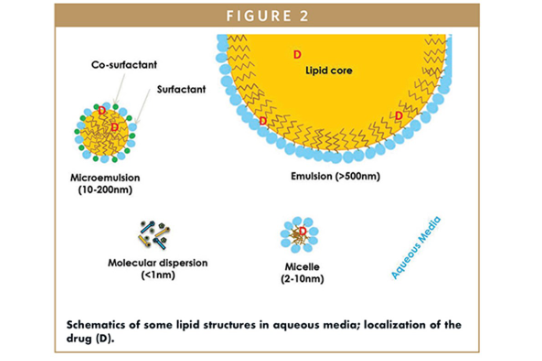
However, lipid formulations, especially softgels, present a compelling alternative that addresses bioavailability issues effectively. By leveraging the body’s natural lipid digestion mechanisms, softgels enhance the solubility and absorption of poorly water-soluble drugs. This not only improves therapeutic outcomes but also reduces the required dosage, which is critical during early development stages when active pharmaceutical ingredient (API) availability may be limited.
Moreover, the semi-continuous manufacturing process of softgels allows for easier scale-up from laboratory to commercial production, streamlining the development timeline. As the pharmaceutical landscape evolves, embracing lipid formulations like softgels can significantly enhance the chances of successful drug development, ultimately leading to better patient outcomes.
Get started today with Solo access →