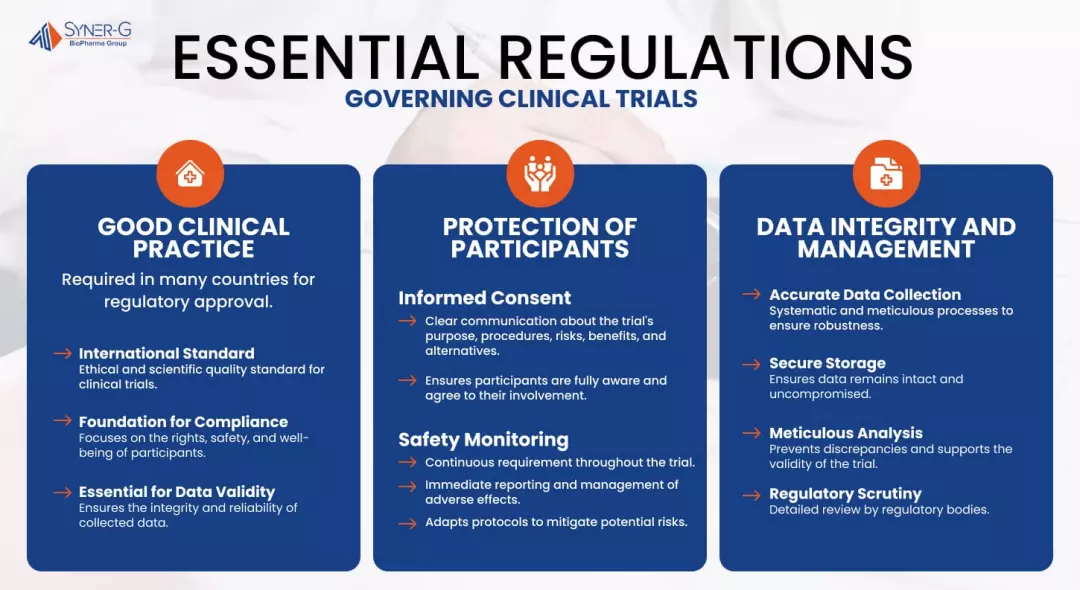
The Medicines and Healthcare products Regulatory Agency (MHRA) has reiterated the critical importance of adhering to Good Clinical Practice (GCP) standards during clinical trials. This emphasis comes as the agency prepares to conduct inspections to ensure compliance among trial sponsors and investigators. The MHRA’s focus on GCP is not merely procedural; it is a fundamental aspect of safeguarding participant welfare and ensuring the integrity of trial data.
In the context of increasing scrutiny on clinical trial conduct, understanding the GCP requirements is essential for pharmaceutical professionals involved in regulatory affairs, quality assurance, and clinical management. The implications of non-compliance can be severe, ranging from delayed approvals to reputational damage. Thus, organizations must prioritize GCP training and comprehensive internal audits to align with MHRA expectations and mitigate risks associated with inspections.
Use the database as your supply chain compass →