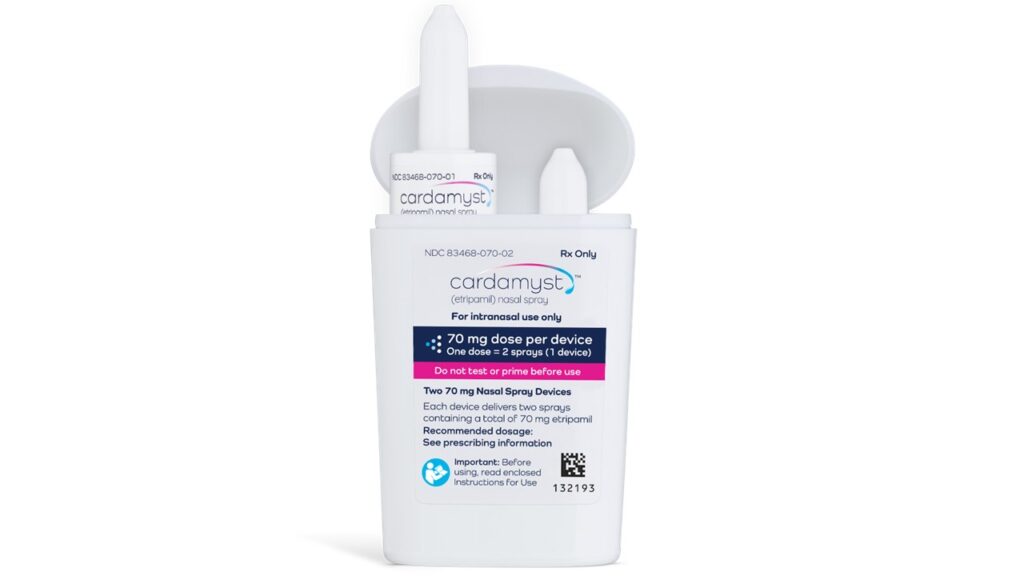
Milestone Pharmaceuticals has achieved a significant regulatory milestone with the FDA’s approval of Cardamyst (etripamil), a nasally administered emergency treatment for paroxysmal supraventricular tachycardia (PSVT), following two previous rejections. This approval marks a turning point for the company, which has faced scrutiny regarding the drug’s efficacy and safety profile.
The approval comes at a time when the demand for innovative, patient-friendly delivery methods in cardiology is on the rise. Cardamyst offers a non-invasive alternative to traditional intravenous therapies, potentially improving patient compliance and outcomes in acute situations. The FDA’s endorsement underscores a growing recognition of the need for accessible treatment options in emergency care.
The implications for Milestone are profound, as this approval not only validates their research and development efforts but also positions them strategically within a competitive market. With the increasing focus on personalized medicine and rapid response therapies, Cardamyst could pave the way for further innovations in the treatment of cardiac conditions.
Start your 7-day trial and see what the database can do →