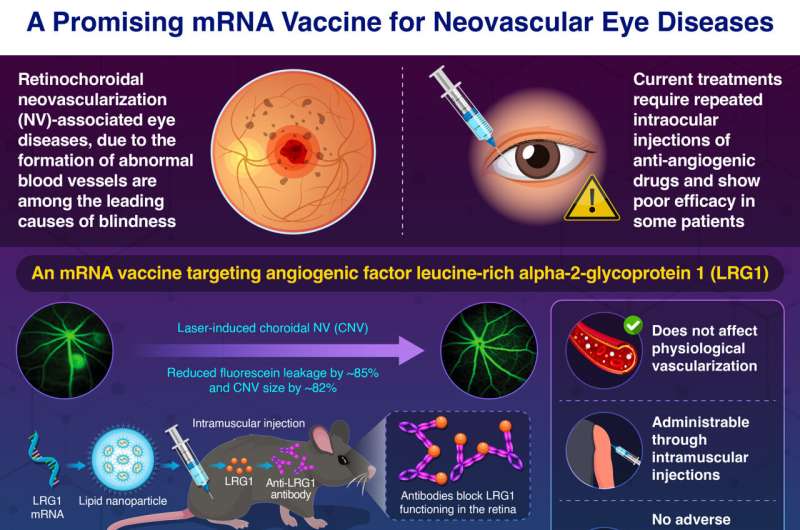
An mRNA vaccine has been shown to effectively suppress neovascularization associated with age-related macular degeneration (AMD) in mouse models, a condition that significantly contributes to vision loss in the elderly. This innovative vaccine can be administered intramuscularly, presenting a more patient-friendly alternative to the current treatment methods, which often involve direct injections into the eye.
Published in Vaccine, the study titled “mRNA vaccination mitigates pathological retinochoroidal neovascularization in animal models.” highlights the potential of mRNA technology, previously validated in COVID-19 vaccines, to address chronic eye diseases. AMD affects nearly 200 million individuals globally, with wet AMD characterized by abnormal blood vessel growth in the retina, leading to fluid leakage and vision impairment. Current treatments require frequent intravitreal injections, which can become burdensome and often lose efficacy over time.
Researchers from the Institute of Science Tokyo, Japan, have developed a novel mRNA vaccine that targets leucine-rich alpha-2-glycoprotein 1 (LRG1), a protein implicated in angiogenesis. The vaccine demonstrated significant efficacy in two mouse models, reducing abnormal blood vessel growth and leakage by up to 85% within weeks of administration. Importantly, the vaccine did not adversely affect normal retinal architecture or provoke harmful immune responses, suggesting a promising safety profile. As noted by lead researcher Satoshi Uchida, MD, PhD, this approach could revolutionize treatment for AMD, offering long-term benefits with a single intramuscular dose, thereby alleviating the treatment burden on patients.
Start your 7-day trial and see what the database can do →