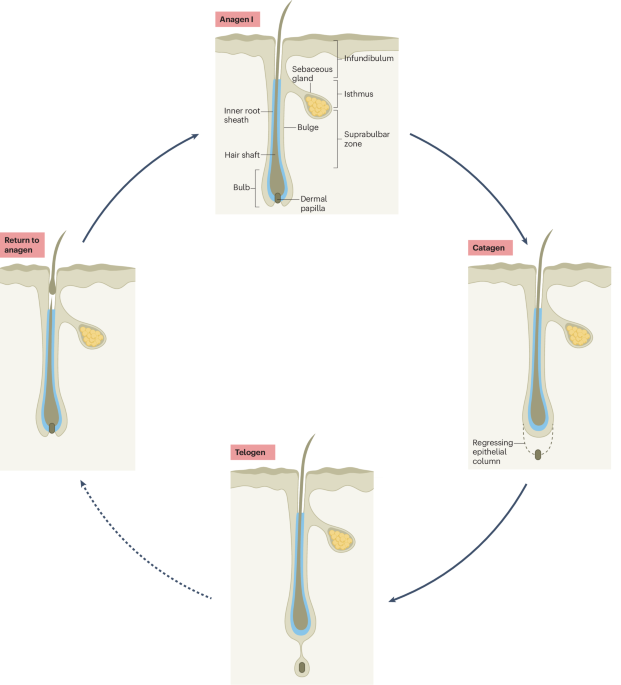
Nektar Therapeutics has announced that its Phase 2b clinical trial for its leading immunology drug targeting severe alopecia did not meet its primary endpoints, a setback that raises questions about the drug’s efficacy. Executives from the company attributed this disappointing outcome to errors in patient enrollment, suggesting that the trial did not adequately represent the target population. Despite this setback, Nektar plans to move forward with the program, indicating confidence in the drug’s potential as they prepare for pivotal testing.
This development highlights the critical importance of precise trial design and patient selection in clinical research, particularly in fields as complex as immunology. The implications for regulatory approval and market entry could be significant, as successful pivotal trials will be essential for demonstrating the drug’s effectiveness and safety to regulatory bodies. As the pharmaceutical industry continues to navigate these challenges, the focus on optimizing enrollment strategies will be paramount for future clinical success.
Open the full market picture for your next decision →