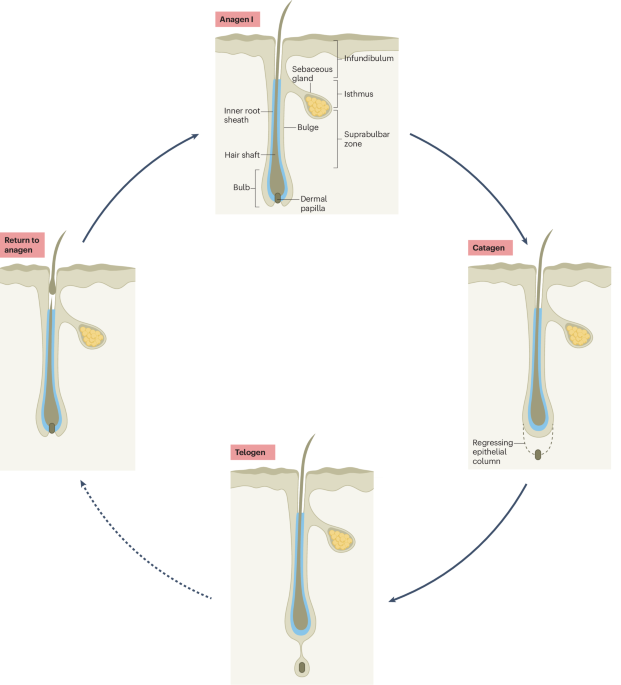
The FDA has announced the modernization of its New Drugs Regulatory Program (NDRP), focusing on the Integrated Assessment of Marketing Applications and Integrated Review Documentation. This initiative aims to streamline the regulatory process for new drug approvals, enhancing efficiency and clarity in the assessment of marketing applications.
As the pharmaceutical industry faces increasing pressure to bring innovative therapies to market swiftly, this modernization effort is designed to reduce bottlenecks and improve communication between regulatory bodies and drug developers. By integrating assessment processes, the FDA seeks to ensure that critical information is readily available and that reviews are conducted in a more cohesive manner.
The implications for B2B professionals in regulatory affairs, quality assurance, and sourcing are significant. This shift could lead to faster approval times and a more predictable regulatory landscape, ultimately impacting portfolio strategies and resource allocation within pharmaceutical companies. Stakeholders are encouraged to engage with the FDA’s request for comments to shape the future of drug regulation.
Use the database as your supply chain compass →