FDA Grants Early Approval for Cytokinetics’ Myqorzo, Marking a Milestone for the Biotech
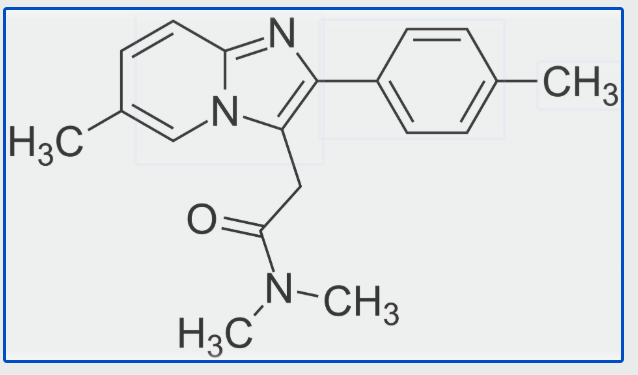
The FDA has granted early approval for Cytokinetics’ aficamten, a significant milestone for the biotech firm as it marks its first U.S. nod since its inception in 1997. The agency’s decision comes ahead of the anticipated December 26 deadline and recognizes aficamten as a treatment for obstructive hypertrophic cardiomyopathy (oHCM), now branded as Myqorzo. This…