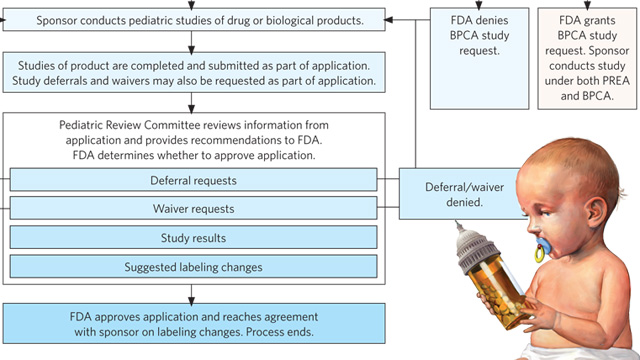
The FDA has released comprehensive reviews of pediatric studies conducted under the Best Pharmaceuticals for Children Act (BPCA) and pediatric assessments performed under the Pediatric Research Equity Act (PREA) from 2012 to the present. These reviews encompass critical medical, statistical, and clinical pharmacology evaluations that aim to enhance the safety and efficacy of pharmaceuticals for pediatric populations.
Contextually, the BPCA and PREA are pivotal legislative frameworks that mandate the assessment of pediatric needs in drug development. The reviews highlight the FDA’s commitment to ensuring that medications are appropriately studied and labeled for children, a demographic often overlooked in clinical trials. By systematically analyzing the outcomes of these studies, the FDA provides valuable insights into the therapeutic landscape for pediatric patients.
The implications of these findings are significant for pharmaceutical companies and regulatory professionals. Understanding the results of these pediatric studies can inform drug development strategies, enhance compliance with regulatory requirements, and ultimately lead to better therapeutic options for children. As the industry continues to prioritize pediatric care, these reviews serve as a critical resource for stakeholders involved in regulatory affairs, quality assurance, and clinical development.
Start your 7-day trial and see what the database can do →