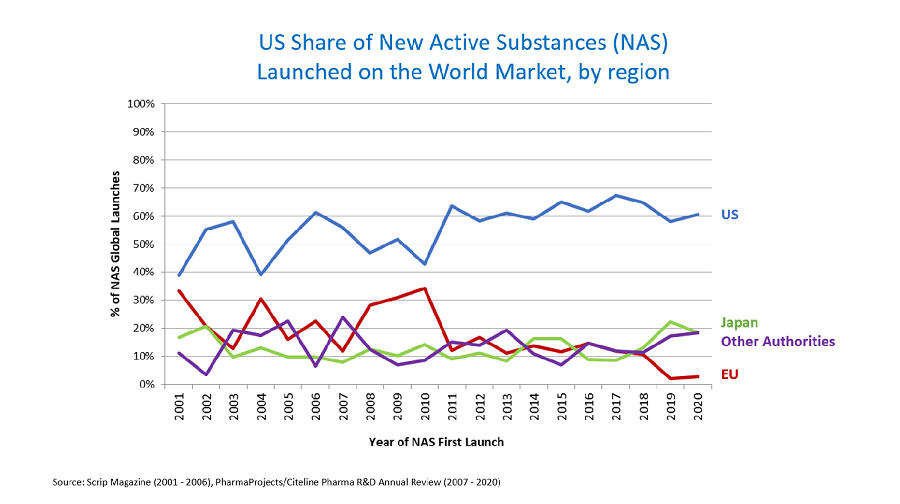
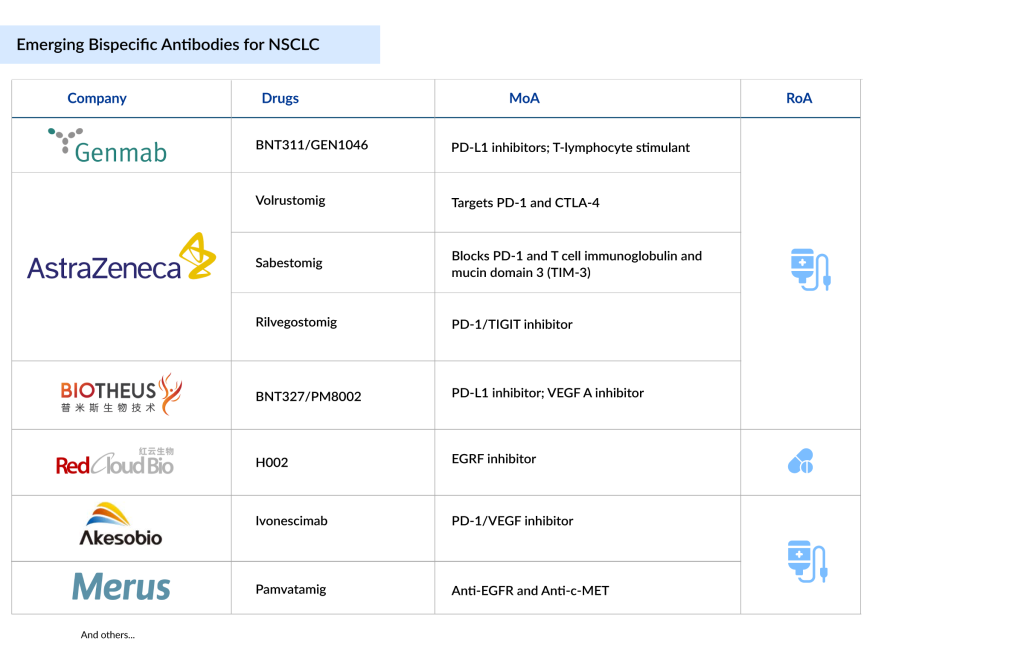
Pfizer has announced that a new formulation of its Covid-19 vaccine has demonstrated a significant increase in antibody levels, achieving at least a four-fold boost in adults. This data comes ahead of the critical respiratory season, underscoring the ongoing need for effective vaccination strategies in the face of evolving variants and public health challenges.
The findings, which were shared in an open-label study, highlight Pfizer’s commitment to enhancing vaccine efficacy as the pandemic continues to impact global health. Collaborating with BioNTech, the co-developer of the Comirnaty vaccine, Pfizer’s latest results may influence vaccination policies and strategies as healthcare professionals prepare for increased respiratory illnesses during the upcoming months.
As regulatory bodies assess this new data, the implications for vaccine distribution and public health initiatives could be substantial. Enhanced antibody responses may lead to broader acceptance of booster shots, ultimately shaping the landscape of Covid-19 vaccination efforts and influencing future vaccine development in the biopharmaceutical sector.
Open the full market picture for your next decision →