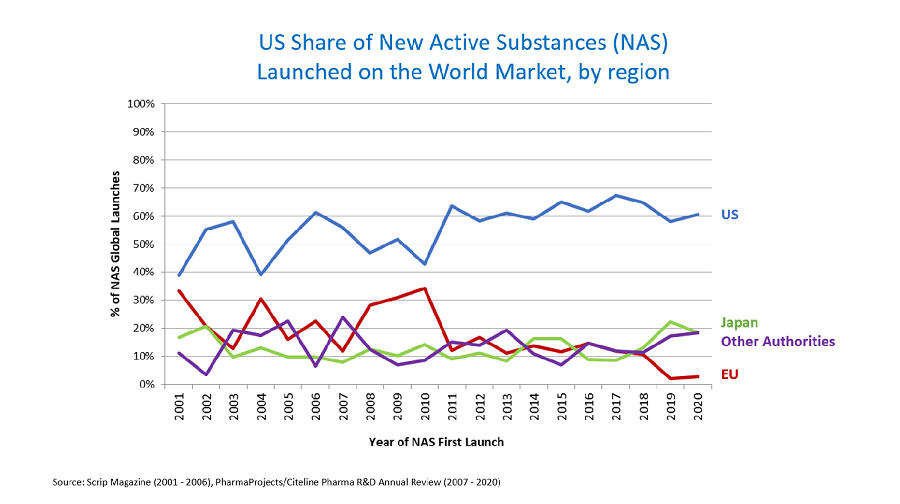
A recent report from Back Bay Life Science Advisors indicates a significant uptick in the testing and investment in multifunctional antibodies that target multiple sites simultaneously. This shift towards a ‘more is better’ strategy reflects the industry’s growing recognition of the limitations of traditional monoclonal antibodies, which typically focus on a single target. The innovative design of these next-generation antibodies aims to enhance therapeutic efficacy, potentially leading to improved patient outcomes in complex diseases such as cancer and autoimmune disorders.
As pharmaceutical companies pivot to this more versatile approach, the implications for regulatory pathways and quality assurance practices are profound. Multifunctional antibodies may require new frameworks for evaluation, necessitating collaboration between regulatory bodies and industry stakeholders to ensure safety and efficacy. Furthermore, the complexity of these products could pose challenges in quality control and manufacturing processes, underscoring the need for robust quality assurance measures throughout the product lifecycle.
Use the database as your supply chain compass →